Sargassum horneri methanol extract rescues C2C12 murine skeletal muscle cells from oxidative stress-induced cytotoxicity through Nrf2-mediated upregulation of heme oxygenase-1
- PMID: 25653022
- PMCID: PMC4324402
- DOI: 10.1186/s12906-015-0538-2
Sargassum horneri methanol extract rescues C2C12 murine skeletal muscle cells from oxidative stress-induced cytotoxicity through Nrf2-mediated upregulation of heme oxygenase-1
Abstract
Background: Sargassum horneri, an edible marine brown alga, is typically distributed along the coastal seas of Korea and Japan. Although several studies have demonstrated the anti-oxidative activity of this alga, the regulatory mechanisms have not yet been defined. The aim of the present study was to examine the cytoprotective effects of S. horneri against oxidative stress-induced cell damage in C2C12 myoblasts.
Methods: We demonstrated the anti-oxidative effects of a methanol extract of S. horneri (SHME) in a hydrogen peroxide (H2O2)-stimulated C2C12 myoblast model. Cytotoxicity was determined using the 3-(4,5-dimetylthiazol-2-yl)-2,5-diphenyl-tetrazolium assay and mode of cell death by cell cycle analysis. DNA damage was measured using a comet assay and expression of phospho-histone γH2A.X (p-γH2A.X). Levels of cellular oxidative stress as reactive oxygen species (ROS) accumulation were measured using 2',7'-dichlorofluorescein diacetate. The involvement of selected genes in the oxidative stress-mediated signaling pathway was explored using Western blot analysis.
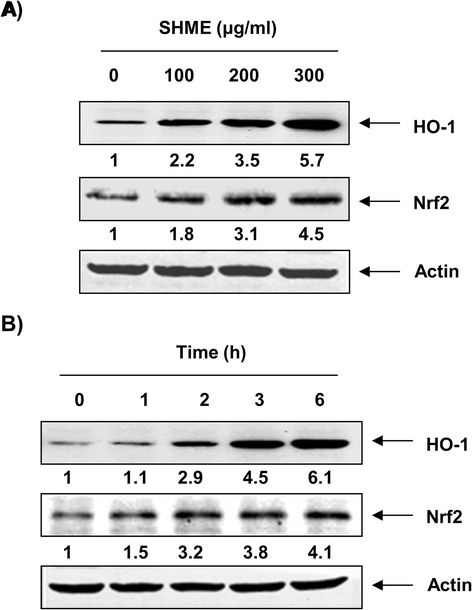
Results: SHME attenuated H2O2-induced growth inhibition and exhibited scavenging activity against intracellular ROS that were induced by H2O2. The SHME also inhibited comet tail formation, p-γH2A.X expression, and the number of sub-G1 hypodiploid cells, suggesting that it prevents H2O2-induced cellular DNA damage and apoptotic cell death. Furthermore, the SHME significantly enhanced the expression of heme oxygenase-1 (HO-1) associated with induction of nuclear factor-erythroid 2 related factor 2 (Nrf2) in a time- and concentration-dependent manner. Moreover, the protective effect of the SHME on H2O2-induced C2C12 cell damage was significantly abolished by zinc protoporphyrin IX, a HO-1 competitive inhibitor, in C2C12 cells.
Conclusions: These findings suggest that the SHME augments cellular antioxidant defense capacity through both intrinsic free radical scavenging activity and activation of the Nrf2/HO-1 pathway, protecting C2C12 cells from H2O2-induced oxidative cytotoxicity.
Figures






Similar articles
-
The Cytoprotective Effect of Petalonia binghamiae Methanol Extract against Oxidative Stress in C2C12 Myoblasts: Mediation by Upregulation of Heme Oxygenase-1 and Nuclear Factor-Erythroid 2 Related Factor 2.Mar Drugs. 2015 Apr 29;13(5):2666-79. doi: 10.3390/md13052666. Mar Drugs. 2015. PMID: 25939035 Free PMC article.
-
The cytoprotective effects of 7,8-dihydroxyflavone against oxidative stress are mediated by the upregulation of Nrf2-dependent HO-1 expression through the activation of the PI3K/Akt and ERK pathways in C2C12 myoblasts.Int J Mol Med. 2015 Aug;36(2):501-10. doi: 10.3892/ijmm.2015.2256. Epub 2015 Jun 22. Int J Mol Med. 2015. PMID: 26096841
-
Schisandrae semen essential oil attenuates oxidative stress-induced cell damage in C2C12 murine skeletal muscle cells through Nrf2‑mediated upregulation of HO‑1.Int J Mol Med. 2015 Feb;35(2):453-9. doi: 10.3892/ijmm.2014.2028. Epub 2014 Dec 8. Int J Mol Med. 2015. PMID: 25482391
-
Nrf2 mediates redox adaptations to exercise.Redox Biol. 2016 Dec;10:191-199. doi: 10.1016/j.redox.2016.10.003. Epub 2016 Oct 14. Redox Biol. 2016. PMID: 27770706 Free PMC article. Review.
-
Exploring the pathophysiological influence of heme oxygenase-1 on neuroinflammation and depression: A study of phytotherapeutic-based modulation.Phytomedicine. 2024 May;127:155466. doi: 10.1016/j.phymed.2024.155466. Epub 2024 Feb 19. Phytomedicine. 2024. PMID: 38461764 Review.
Cited by
-
The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes.J Muscle Res Cell Motil. 2015 Dec;36(6):377-93. doi: 10.1007/s10974-015-9438-9. Epub 2016 Jan 4. J Muscle Res Cell Motil. 2015. PMID: 26728750 Free PMC article. Review.
-
Adiponectin modulates oxidative stress-induced mitophagy and protects C2C12 myoblasts against apoptosis.Sci Rep. 2017 Jun 9;7(1):3209. doi: 10.1038/s41598-017-03319-2. Sci Rep. 2017. PMID: 28600493 Free PMC article.
-
Antifibrosis Efficacy of Apo-9-Fucoxanthinone-Contained Sargassum horneri Ethanol Extract on Nasal Polyp: An In Vitro and Ex Vivo Organ Culture Assay.Curr Issues Mol Biol. 2022 Nov 21;44(11):5815-5826. doi: 10.3390/cimb44110395. Curr Issues Mol Biol. 2022. PMID: 36421679 Free PMC article.
-
Curcumin Ameliorated Oxidative Stress and Inflammation-Related Muscle Disorders in C2C12 Myoblast Cells.Antioxidants (Basel). 2021 Mar 17;10(3):476. doi: 10.3390/antiox10030476. Antioxidants (Basel). 2021. PMID: 33802935 Free PMC article.
-
The Effects of Sargassum horneri Extract and Fucoidan on Tear Hyposecretion and Ocular Surface Injury in Rats with Dry Eye Diseases.Curr Issues Mol Biol. 2023 Aug 8;45(8):6583-6592. doi: 10.3390/cimb45080415. Curr Issues Mol Biol. 2023. PMID: 37623234 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

