A novel combinational approach of microstimulation and bioluminescence imaging to study the mechanisms of action of cerebral electrical stimulation in mice
- PMID: 25653107
- PMCID: PMC4457191
- DOI: 10.1113/jphysiol.2014.287243
A novel combinational approach of microstimulation and bioluminescence imaging to study the mechanisms of action of cerebral electrical stimulation in mice
Abstract
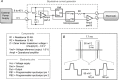
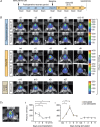
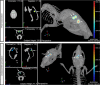
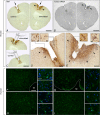
Deep brain stimulation (DBS) is used to treat a number of neurological conditions and is currently being tested to intervene in neuropsychiatric conditions. However, a better understanding of how it works would ensure that side effects could be minimized and benefits optimized. We have thus developed a unique device to perform brain stimulation (BS) in mice and to address fundamental issues related to this methodology in the pre-clinical setting. This new microstimulator prototype was specifically designed to allow simultaneous live bioluminescence imaging of the mouse brain, allowing real time assessment of the impact of stimulation on cerebral tissue. We validated the authenticity of this tool in vivo by analysing the expression of toll-like receptor 2 (TLR2), corresponding to the microglial response, in the stimulated brain regions of TLR2-fluc-GFP transgenic mice, which we further corroborated with post-mortem analyses in these animals as well as in human brains of patients who underwent DBS to treat their Parkinson's disease. In the present study, we report on the development of the first BS device that allows for simultaneous live in vivo imaging in mice. This tool opens up a whole new range of possibilities that allow a better understanding of BS and how to optimize its effects through its use in murine models of disease.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures


References
-
- Ackermans L, Duits A, Linden Cvd, Tijssen M, Schruers K, Temel Y, Kleijer M, Nederveen P, Bruggeman R, Tromp S, Kranen-Mastenbroek Vv, Kingma H, Cath D. Visser-Vandewalle V. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain. 2011;134:832–844. - PubMed
-
- Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A, Fabrigoule C, Allard M, Rougier A, Bioulac B, Tignol J. Burbaud P. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg. 2004;101:682–686. - PubMed
-
- Azevedo EP, Ledo JH, Barbosa G, Sobrinho M, Diniz L, Fonseca AC, Gomes F, Romao L, Lima FR, Palhano FL, Ferreira ST. Foguel D. Activated microglia mediate synapse loss and short-term memory deficits in a mouse model of transthyretin-related oculoleptomeningeal amyloidosis. Cell Death Dis. 2013;4:e789. - PMC - PubMed
-
- Baba T, Kameda M, Yasuhara T, Morimoto T, Kondo A, Shingo T, Tajiri N, Wang F, Miyoshi Y, Borlongan CV, Matsumae M. Date I. Electrical stimulation of the cerebral cortex exerts antiapoptotic, angiogenic, and anti-inflammatory effects in ischemic stroke rats through phosphoinositide 3-kinase/Akt signaling pathway. Stroke. 2009;40:e598–e605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

