Quantitative high throughput screening using a primary human three-dimensional organotypic culture predicts in vivo efficacy
- PMID: 25653139
- PMCID: PMC4427252
- DOI: 10.1038/ncomms7220
Quantitative high throughput screening using a primary human three-dimensional organotypic culture predicts in vivo efficacy
Erratum in
-
Erratum: Quantitative high throughput screening using a primary human three-dimensional organotypic culture predicts in vivo efficacy.Nat Commun. 2016 Feb 3;7:10649. doi: 10.1038/ncomms10649. Nat Commun. 2016. PMID: 26838815 Free PMC article. No abstract available.
Abstract
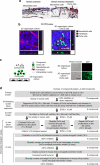
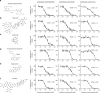
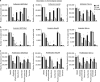
The tumour microenvironment contributes to cancer metastasis and drug resistance. However, most high throughput screening (HTS) assays for drug discovery use cancer cells grown in monolayers. Here we show that a multilayered culture containing primary human fibroblasts, mesothelial cells and extracellular matrix can be adapted into a reliable 384- and 1,536-multi-well HTS assay that reproduces the human ovarian cancer (OvCa) metastatic microenvironment. We validate the identified inhibitors in secondary in vitro and in vivo biological assays using three OvCa cell lines: HeyA8, SKOV3ip1 and Tyk-nu. The active compounds directly inhibit at least two of the three OvCa functions: adhesion, invasion and growth. In vivo, these compounds prevent OvCa adhesion, invasion and metastasis, and improve survival in mouse models. Collectively, these data indicate that a complex three-dimensional culture of the tumour microenvironment can be adapted for quantitative HTS and may improve the disease relevance of assays used for drug screening.
Figures


References
-
- Hanahan D, Coussens LM. Accessories to the crime: functions of cell recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. - PubMed
-
- Zang R, Ding L, Tang IC, Wang J, Yang ST. Cell-based assays in high-throughput screening for drug discovery. Int. J. Biotechnol. Wellness Ind. 2012;1:31–51.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

