The KCNQ1 channel - remarkable flexibility in gating allows for functional versatility
- PMID: 25653179
- PMCID: PMC4500346
- DOI: 10.1113/jphysiol.2014.287607
The KCNQ1 channel - remarkable flexibility in gating allows for functional versatility
Abstract
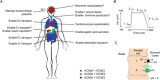
The KCNQ1 channel (also called Kv7.1 or KvLQT1) belongs to the superfamily of voltage-gated K(+) (Kv) channels. KCNQ1 shares several general features with other Kv channels but also displays a fascinating flexibility in terms of the mechanism of channel gating, which allows KCNQ1 to play different physiological roles in different tissues. This flexibility allows KCNQ1 channels to function as voltage-independent channels in epithelial tissues, whereas KCNQ1 function as voltage-activated channels with very slow kinetics in cardiac tissues. This flexibility is in part provided by the association of KCNQ1 with different accessory KCNE β-subunits and different modulators, but also seems like an integral part of KCNQ1 itself. The aim of this review is to describe the main mechanisms underlying KCNQ1 flexibility.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures
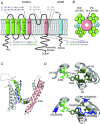

References
-
- Abbott GW. Biology of the KCNQ1 potassium channel. New J Sci. 2014;2014:237431.
-
- Anantharam A, Markowitz SM. Abbott GW. Pharmacogenetic considerations in diseases of cardiac ion channels. J Pharmacol Exp Ther. 2003;307:831–838. - PubMed
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M. Romey G. KVLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Barrett KE. Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

