Local iontophoretic administration of cytotoxic therapies to solid tumors
- PMID: 25653220
- PMCID: PMC4545246
- DOI: 10.1126/scitranslmed.3009951
Local iontophoretic administration of cytotoxic therapies to solid tumors
Abstract
Parenteral and oral routes have been the traditional methods of administering cytotoxic agents to cancer patients. Unfortunately, the maximum potential effect of these cytotoxic agents has been limited because of systemic toxicity and poor tumor perfusion. In an attempt to improve the efficacy of cytotoxic agents while mitigating their side effects, we have developed modalities for the localized iontophoretic delivery of cytotoxic agents. These iontophoretic devices were designed to be implanted proximal to the tumor with external control of power and drug flow. Three distinct orthotopic mouse models of cancer and a canine model were evaluated for device efficacy and toxicity. Orthotopic patient-derived pancreatic cancer xenografts treated biweekly with gemcitabine via the device for 7 weeks experienced a mean log2 fold change in tumor volume of -0.8 compared to a mean log2 fold change in tumor volume of 1.1 for intravenous (IV) gemcitabine, 3.0 for IV saline, and 2.6 for device saline groups. The weekly coadministration of systemic cisplatin therapy and transdermal device cisplatin therapy significantly increased tumor growth inhibition and doubled the survival in two aggressive orthotopic models of breast cancer. The addition of radiotherapy to this treatment further extended survival. Device delivery of gemcitabine in dogs resulted in more than 7-fold difference in local drug concentrations and 25-fold lower systemic drug levels than the IV treatment. Overall, these devices have potential paradigm shifting implications for the treatment of pancreatic, breast, and other solid tumors.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures

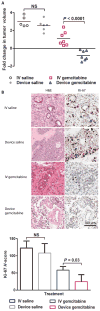


References
-
- Goodman LS, Wintrobe MM, Dameshek W, Goodman MJ, Gilman MA, McLennan MT. Nitrogen mustard therapy; use of methyl-bis (β-chloroethyl) amine hydrochloride and tris (β-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J Am Med Assoc. 1946;132:126–132. - PubMed
-
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler; MW European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. - PubMed
-
- Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab; R German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. - PubMed
-
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua; YJ MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. - PubMed
-
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman; AR Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

