Antiepileptic drug use by pregnant women enrolled in Florida Medicaid
- PMID: 25653296
- PMCID: PMC4351665
- DOI: 10.1212/WNL.0000000000001304
Antiepileptic drug use by pregnant women enrolled in Florida Medicaid
Abstract
Objective: The study aims were to investigate secular trends in antiepileptic drug (AED) use in women during pregnancy, and to compare the use of first- and second-generation AEDs.
Methods: Study participants consisted of female Florida Medicaid beneficiaries, older than 15 years, and pregnant within the time period 1999 to 2009. Fifteen AEDs were categorized into first and second generation of AEDs. Continuous use of AEDs was defined as at least 2 consecutive AED prescriptions totaling more than a 30-day supply. Polytherapy was defined as 2 or more AEDs continuously used for at least 30 overlapping days. Annual prevalence was estimated and compared.
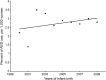
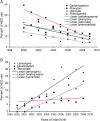
Results: We included 2,099 pregnant women who were enrolled in Florida Medicaid from 1999 to 2009 and exposed to AEDs during pregnancy. Although there were fluctuations, overall AED use in the study cohort did not increase from 2000 to 2009 (β ± standard error [SE]: -0.07 ± 0.06, p = 0.31). The use of first-generation AEDs decreased (β ± SE: -6.21 ± 0.47, p < 0.0001), whereas the use of second-generation AEDs increased (β ± SE: 6.27 ± 0.52, p < 0.0001) from 2000 to 2009. AED use in polytherapy did not change through the study period. Valproate use reduced from 23% to 8% in the study population (β ± SE: -1.61 ± 0.36, p = 0.0019), but this decrease was only for women receiving an AED for epilepsy and was not present for other indications.
Conclusion: The second-generation AEDs are replacing first-generation AEDs in both monotherapy and polytherapy. Valproate use has declined for epilepsy but not other indications. Additional changes in AED use are expected in future years.
© 2015 American Academy of Neurology.
Figures


Comment in
-
Comment: Are we filling knowledge gaps about antiepileptic drugs and pregnancy?Neurology. 2015 Mar 3;84(9):950. doi: 10.1212/WNL.0000000000001320. Epub 2015 Feb 4. Neurology. 2015. PMID: 25653293 No abstract available.
References
-
- Samren EB, van Duijn CM, Christianens GC, et al. Antiepileptic drug regimens and major congenital abnormalities in the offspring. Ann Neurol 1999;46:739–746. - PubMed
-
- Kaneko S, Kondo T. Antiepileptic agents and birth defects: incidence, mechanisms and prevention. CNS Drugs 1995;3:41–55.
-
- Tomson T. Which drug for the pregnant woman with epilepsy? N Engl J Med 2009;360:1667–1669. - PubMed
-
- Kaneko S, Otani K, Fukushima Y, et al. Teratogenicity of antiepileptic drugs: analysis of possible risk factors. Epilepsia 1988;29:459–467. - PubMed
-
- Friis ML. Facial clefts and congenital heart defects in children of parents with epilepsy: genetic and environmental etiologic factors. Acta Neurol Scand 1989;79:433–459. - PubMed
