Developmental tightening of cerebellar cortical synaptic influx-release coupling
- PMID: 25653347
- PMCID: PMC6705354
- DOI: 10.1523/JNEUROSCI.2900-14.2015
Developmental tightening of cerebellar cortical synaptic influx-release coupling
Abstract
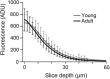
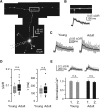
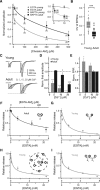
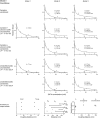
Tight coupling between Ca(2+) channels and the sensor for vesicular transmitter release at the presynaptic active zone (AZ) is crucial for high-fidelity synaptic transmission. It has been hypothesized that a switch from a loosely coupled to a tightly coupled transmission mode is a common step in the maturation of CNS synapses. However, this hypothesis has never been tested at cortical synapses. We addressed this hypothesis at a representative small cortical synapse: the synapse connecting mouse cerebellar cortical parallel fibers to Purkinje neurons. We found that the slow Ca(2+) chelator EGTA affected release significantly stronger at immature than at mature synapses, while the fast chelator BAPTA was similarly effective in both groups. Analysis of paired-pulse ratios and quantification of release probability (pr) with multiple-probability fluctuation analysis revealed increased facilitation at immature synapses accompanied by reduced pr. Cav2.1 Ca(2+) channel immunoreactivity, assessed by quantitative high-resolution immuno-electron microscopy, was scattered over immature boutons but confined to putative AZs at mature boutons. Presynaptic Ca(2+) signals were quantified with two-photon microscopy and found to be similar between maturation stages. Models adjusted to fit EGTA dose-response curves as well as differential effects of the Ca(2+) channel blocker Cd(2+) indicate looser and less homogenous coupling at immature terminals compared with mature ones. These results demonstrate functionally relevant developmental tightening of influx-release coupling at a single AZ cortical synapse and corroborate developmental tightening of coupling as a prevalent phenomenon in the mammalian brain.
Keywords: calcium channels; calcium chelators; coupling; presynaptic calcium; release probability; short-term plasticity.
Copyright © 2015 the authors 0270-6474/15/351858-14$15.00/0.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
