B-lymphocyte-mediated delayed cognitive impairment following stroke
- PMID: 25653369
- PMCID: PMC4315838
- DOI: 10.1523/JNEUROSCI.4098-14.2015
B-lymphocyte-mediated delayed cognitive impairment following stroke
Abstract
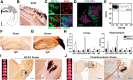
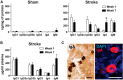
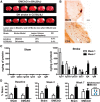
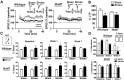
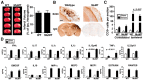
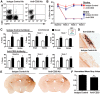
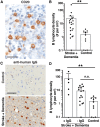
Each year, 10 million people worldwide survive the neurologic injury associated with a stroke. Importantly, stroke survivors have more than twice the risk of subsequently developing dementia compared with people who have never had a stroke. The link between stroke and the later development of dementia is not understood. There are reports of oligoclonal bands in the CSF of stroke patients, suggesting that in some people a B-lymphocyte response to stroke may occur in the CNS. Therefore, we tested the hypothesis that a B-lymphocyte response to stroke could contribute to the onset of dementia. We discovered that, in mouse models, activated B-lymphocytes infiltrate infarcted tissue in the weeks after stroke. B-lymphocytes undergo isotype switching, and IgM, IgG, and IgA antibodies are found in the neuropil adjacent to the lesion. Concurrently, mice develop delayed deficits in LTP and cognition. Genetic deficiency, and the pharmacologic ablation of B-lymphocytes using an anti-CD20 antibody, prevents the appearance of delayed cognitive deficits. Furthermore, immunostaining of human postmortem tissue revealed that a B-lymphocyte response to stroke also occurs in the brain of some people with stroke and dementia. These data suggest that some stroke patients may develop a B-lymphocyte response to stroke that contributes to dementia, and is potentially treatable with FDA-approved drugs that target B cells.
Keywords: B-lymphocyte; dementia; immunology; stroke.
Copyright © 2015 the authors 0270-6474/15/352133-13$15.00/0.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
B cells: Meddling with the mind.Nat Rev Immunol. 2015 Mar;15(3):135. doi: 10.1038/nri3826. Epub 2015 Feb 20. Nat Rev Immunol. 2015. PMID: 25698677 No abstract available.
-
Stroke: Meddling with the mind.Nat Rev Drug Discov. 2015 Mar;14(3):166. doi: 10.1038/nrd4561. Nat Rev Drug Discov. 2015. PMID: 25722242 No abstract available.
References
-
- Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci U S A. 2011;108:13287–13292. doi: 10.1073/pnas.1107368108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
