CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus (SIV) vaccine enhances protection against neutralization-resistant mucosal SIV infection
- PMID: 25653428
- PMCID: PMC4442387
- DOI: 10.1128/JVI.03527-14
CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus (SIV) vaccine enhances protection against neutralization-resistant mucosal SIV infection
Abstract
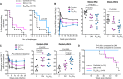
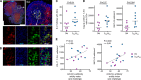
Here, we show that a CD40L-adjuvanted DNA/modified vaccinia virus Ankara (MVA) simian immunodeficiency virus (SIV) vaccine enhances protection against a pathogenic neutralization-resistant mucosal SIV infection, improves long-term viral control, and prevents AIDS. Analyses of serum IgG antibodies to linear peptides of SIV Env revealed a strong response to V2, with targeting of fewer epitopes in the immunodominant region of gp41 (gp41-ID) and the V1 region as a correlate for enhanced protection. Greater expansion of antiviral CD8 T cells in the gut correlated with long-term viral control.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Kwa S, Lai L, Gangadhara S, Siddiqui M, Pillai VB, Labranche C, Yu T, Moss B, Montefiori DC, Robinson HL, Kozlowski PA, Amara RR. 2014. CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge. J Virol 88:9579–9589. doi:10.1128/JVI.00975-14. - DOI - PMC - PubMed
-
- Lopker M, Easlick J, Sterrett S, Decker JM, Barbian H, Learn G, Keele BF, Robinson JE, Li H, Hahn BH, Shaw GM, Bar KJ. 2013. Heterogeneity in neutralization sensitivities of viruses comprising the simian immunodeficiency virus SIVsmE660 isolate and vaccine challenge stock. J Virol 87:5477–5492. doi:10.1128/JVI.03419-12. - DOI - PMC - PubMed
-
- Kannanganat S, Nigam P, Velu V, Earl PL, Lai L, Chennareddi L, Lawson B, Wilson RL, Montefiori DC, Kozlowski PA, Moss B, Robinson HL, Amara RR. 2010. Preexisting vaccinia virus immunity decreases SIV-specific cellular immunity but does not diminish humoral immunity and efficacy of a DNA/MVA vaccine. J Immunol 185:7262–7273. doi:10.4049/jimmunol.1000751. - DOI - PMC - PubMed
-
- Van Rompay KK, Greenier JL, Cole KS, Earl P, Moss B, Steckbeck JD, Pahar B, Rourke T, Montelaro RC, Canfield DR, Tarara RP, Miller C, McChesney MB, Marthas ML. 2003. Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251. J Virol 77:179–190. doi:10.1128/JVI.77.1.179-190.2003. - DOI - PMC - PubMed
-
- National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

