Dynamics of the cytotoxic T cell response to a model of acute viral infection
- PMID: 25653453
- PMCID: PMC4442358
- DOI: 10.1128/JVI.03474-14
Dynamics of the cytotoxic T cell response to a model of acute viral infection
Abstract
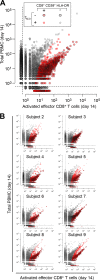
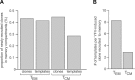
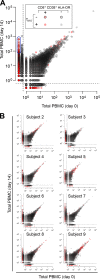
A detailed characterization of the dynamics and breadth of the immune response to an acute viral infection, as well as the determinants of recruitment to immunological memory, can greatly contribute to our basic understanding of the mechanics of the human immune system and can ultimately guide the design of effective vaccines. In addition to neutralizing antibodies, T cells have been shown to be critical for the effective resolution of acute viral infections. We report the first in-depth analysis of the dynamics of the CD8(+) T cell repertoire at the level of individual T cell clonal lineages upon vaccination of human volunteers with a single dose of YF-17D. This live attenuated yellow fever virus vaccine yields sterile, long-term immunity and has been previously used as a model to understand the immune response to a controlled acute viral infection. We identified and enumerated unique CD8(+) T cell clones specifically induced by this vaccine through a combined experimental and statistical approach that included high-throughput sequencing of the CDR3 variable region of the T cell receptor β-chain and an algorithm that detected significantly expanded T cell clones. This allowed us to establish that (i) on average, ∼ 2,000 CD8(+) T cell clones were induced by YF-17D, (ii) 5 to 6% of the responding clones were recruited to long-term memory 3 months postvaccination, (iii) the most highly expanded effector clones were preferentially recruited to the memory compartment, and (iv) a fraction of the YF-17D-induced clones could be identified from peripheral blood lymphocytes solely by measuring clonal expansion.
Importance: The exhaustive investigation of pathogen-induced effector T cells is essential to accurately quantify the dynamics of the human immune response. The yellow fever vaccine (YFV) has been broadly used as a model to understand how a controlled, self-resolving acute viral infection induces an effective and long-term protective immune response. Here, we extend this previous work by reporting the identity of activated effector T cell clones that expand in response to the YFV 2 weeks postvaccination (as defined by their unique T cell receptor gene sequence) and by tracking clones that enter the memory compartment 3 months postvaccination. This is the first study to use high-throughput sequencing of immune cells to characterize the breadth of the antiviral effector cell response and to determine the contribution of unique virus-induced clones to the long-lived memory T cell repertoire. Thus, this study establishes a benchmark against which future vaccines can be compared to predict their efficacy.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

