Proteasome inhibitor bortezomib suppresses nuclear factor-kappa B activation and ameliorates eye inflammation in experimental autoimmune uveitis
- PMID: 25653480
- PMCID: PMC4306382
- DOI: 10.1155/2015/847373
Proteasome inhibitor bortezomib suppresses nuclear factor-kappa B activation and ameliorates eye inflammation in experimental autoimmune uveitis
Abstract
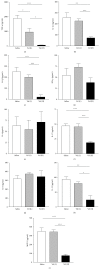
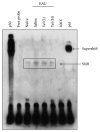
Bortezomib is a proteasome inhibitor used for hematologic cancer treatment. Since it can suppress NF-κB activation, which is critical for the inflammatory process, bortezomib has been found to possess anti-inflammatory activity. In this study, we evaluated the effect of bortezomib on experimental autoimmune uveitis (EAU) in mice and investigated the potential mechanisms related to NF-κB inactivation. High-dose bortezomib (0.75 mg/kg), low-dose bortezomib (0.15 mg/kg), or phosphate buffered saline was given after EAU induction. We found that the EAU is ameliorated by high-dose bortezomib treatment when compared with low-dose bortezomib or PBS treatment. The DNA-binding activity of NF-κB was suppressed and expression of several key inflammatory mediators including TNF-α, IL-1α, IL-1β, IL-12, IL-17, and MCP-1 was lowered in the high-dose bortezomib-treated group. These results suggest that proteasome inhibition is a promising treatment strategy for autoimmune uveitis.
Figures


Similar articles
-
Anti-inflammatory effect of the proteasome inhibitor bortezomib on endotoxin-induced uveitis in rats.Invest Ophthalmol Vis Sci. 2012 Jun 26;53(7):3682-94. doi: 10.1167/iovs.12-9505. Invest Ophthalmol Vis Sci. 2012. PMID: 22538426
-
Chitosan Oligosaccharides Suppress Nuclear Factor-Kappa B Activation and Ameliorate Experimental Autoimmune Uveoretinitis in Mice.Int J Mol Sci. 2020 Nov 6;21(21):8326. doi: 10.3390/ijms21218326. Int J Mol Sci. 2020. PMID: 33171990 Free PMC article.
-
Amelioration of experimental autoimmune uveoretinitis (EAU) with an inhibitor of nuclear factor-kappaB (NF-kappaB), pyrrolidine dithiocarbamate.J Leukoc Biol. 2006 Jun;79(6):1193-201. doi: 10.1189/jlb.0805453. Epub 2006 Mar 30. J Leukoc Biol. 2006. PMID: 16574770
-
[Therapeutic effect of the low molecular weight inhibitor of the NF-kappaB signaling pathway on experimental autoimmune uveoretinitis].Nippon Ganka Gakkai Zasshi. 2010 Nov;114(11):944-54. Nippon Ganka Gakkai Zasshi. 2010. PMID: 21141074 Review. Japanese.
-
Proteasome inhibitors as experimental therapeutics of autoimmune diseases.Arthritis Res Ther. 2015 Jan 28;17(1):17. doi: 10.1186/s13075-015-0529-1. Arthritis Res Ther. 2015. PMID: 25889583 Free PMC article. Review.
Cited by
-
Suppression of the Reactive Oxygen Response Alleviates Experimental Autoimmune Uveitis in Mice.Int J Mol Sci. 2020 May 5;21(9):3261. doi: 10.3390/ijms21093261. Int J Mol Sci. 2020. PMID: 32380695 Free PMC article.
-
Retinal metabolic events in preconditioning light stress as revealed by wide-spectrum targeted metabolomics.Metabolomics. 2017;13(3):22. doi: 10.1007/s11306-016-1156-9. Epub 2017 Jan 20. Metabolomics. 2017. PMID: 28706468 Free PMC article.
-
Targeting the integrated stress response in ophthalmology.Curr Eye Res. 2021 Aug;46(8):1075-1088. doi: 10.1080/02713683.2020.1867748. Epub 2021 Jan 21. Curr Eye Res. 2021. PMID: 33474991 Free PMC article. Review.
-
Preclinical evaluation of antitumor activity of the proteasome inhibitor MLN2238 (ixazomib) in hepatocellular carcinoma cells.Cell Death Dis. 2018 Jan 18;9(2):28. doi: 10.1038/s41419-017-0195-0. Cell Death Dis. 2018. PMID: 29348495 Free PMC article.
-
Bortezomib inhibits proliferation, migration, and TGF-β1-induced epithelial-mesenchymal transition of RPE cells.Mol Vis. 2017 Dec 29;23:1029-1038. eCollection 2017. Mol Vis. 2017. PMID: 29386876 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

