Phenotypic and functional analysis of HL-60 cells used in opsonophagocytic-killing assay for Streptococcus pneumoniae
- PMID: 25653484
- PMCID: PMC4310939
- DOI: 10.3346/jkms.2015.30.2.145
Phenotypic and functional analysis of HL-60 cells used in opsonophagocytic-killing assay for Streptococcus pneumoniae
Abstract
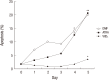
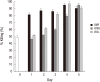
Differentiated HL-60 is an effector cell widely used for the opsonophagocytic-killing assay (OPKA) to measure efficacy of pneumococcal vaccines. We investigated the correlation between phenotypic expression of immunoreceptors and phagocytic ability of HL-60 cells differentiated with N,N-dimethylformamide (DMF), all-trans retinoic acid (ATRA), or 1α, 25-dihydroxyvitamin D3 (VitD3) for 5 days. Phenotypic change was examined by flow cytometry with specific antibodies to CD11c, CD14, CD18, CD32, and CD64. Apoptosis was determined by flow cytometry using 7-aminoactinomycin D. Function was evaluated by a standard OPKA against serotype 19F and chemiluminescence-based respiratory burst assay. The expression of CD11c and CD14 gradually increased upon exposure to all three agents, while CD14 expression increased abruptly after VitD3. The expression of CD18, CD32, and CD64 increased during differentiation with all three agents. Apoptosis remained less than 10% until day 3 but increased after differentiation by DMF or ATRA. Differentiation with ATRA or VitD3 increased the respiratory burst after day 4. DMF differentiation showed a high OPKA titer at day 1 which sustained thereafter while ATRA or VitD3-differentiated cells gradually increased. Pearson analysis between the phenotypic changes and OPKA titers suggests that CD11c might be a useful differentiation marker for HL-60 cells for use in pneumococcal OPKA.
Keywords: Differentiation; HL-60; Opsonophagocytic-Killing Assay; Streptococcus pneumonia.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Centers for Disease Control (CDC) Pneumococcal polysaccharide vaccine. MMWR Morb Mortal Wkly Rep. 1989;38:64–68. 73–76. - PubMed
-
- Fedson DS, Musher DM. Pneumococcal polysaccharide vaccine. In: Plotkin SA, Orenstein WA, editors. Vaccines. 4th ed. Philadelphia: Pa: Saunders; 2004.
-
- Whitney CG, Farley MM, Hadler J, Harrison LH, Lexau C, Reingold A, Lefkowitz L, Cieslak PR, Cetron M, Zell ER, et al. Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343:1917–1924. - PubMed
-
- Tan TQ. Antibiotic resistant infections due to Streptococcus pneumoniae: impact on therapeutic options and clinical outcome. Curr Opin Infect Dis. 2003;16:271–277. - PubMed
-
- Siber GR. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

