Empirical modeling of the fine particle fraction for carrier-based pulmonary delivery formulations
- PMID: 25653522
- PMCID: PMC4310720
- DOI: 10.2147/IJN.S75758
Empirical modeling of the fine particle fraction for carrier-based pulmonary delivery formulations
Abstract
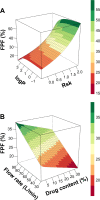
In vitro study of the deposition of drug particles is commonly used during development of formulations for pulmonary delivery. The assay is demanding, complex, and depends on: properties of the drug and carrier particles, including size, surface characteristics, and shape; interactions between the drug and carrier particles and assay conditions, including flow rate, type of inhaler, and impactor. The aerodynamic properties of an aerosol are measured in vitro using impactors and in most cases are presented as the fine particle fraction, which is a mass percentage of drug particles with an aerodynamic diameter below 5 μm. In the present study, a model in the form of a mathematical equation was developed for prediction of the fine particle fraction. The feature selection was performed using the R-environment package "fscaret". The input vector was reduced from a total of 135 independent variables to 28. During the modeling stage, techniques like artificial neural networks, genetic programming, rule-based systems, and fuzzy logic systems were used. The 10-fold cross-validation technique was used to assess the generalization ability of the models created. The model obtained had good predictive ability, which was confirmed by a root-mean-square error and normalized root-mean-square error of 4.9 and 11%, respectively. Moreover, validation of the model using external experimental data was performed, and resulted in a root-mean-square error and normalized root-mean-square error of 3.8 and 8.6%, respectively.
Keywords: deposition modeling; empirical modeling; feature selection; fine particle fraction; genetic programming; pulmonary delivery.
Figures




References
-
- Pilcer G, Wauthoz N, Amighi K. Lactose characteristics and the generation of the aerosol. Adv Drug Deliv Rev. 2012;64(3):233–256. - PubMed
-
- Hassan MS, Lau R. Inhalation performance of pollen-shape carrier in dry powder formulation: effect of size and surface morphology. Int J Pharm. 2011;413(1–2):93–102. - PubMed
-
- Chinga G, Johnsen PO, Dougherty R, Berli EL, Walter J. Quantification of the 3D microstructure of SC surfaces. J Microsc. 2007;227(Pt 3):254–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

