A comparison of letrozole and anastrozole followed by letrozole in breast cancer patients
- PMID: 25653555
- PMCID: PMC4309671
- DOI: 10.2147/BCTT.S73997
A comparison of letrozole and anastrozole followed by letrozole in breast cancer patients
Abstract
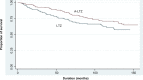
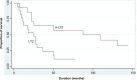
Background: We previously studied the noninferiority of anastrozole (ANZ) versus ANZ followed by letrozole (A-LTZ) due to reimbursement policy. We found that patients with A-LTZ had better overall survival (OS) than did patients with ANZ alone. This study aimed to prove that patients with A-LTZ also had better OS than patients with letrozole (LTZ) alone.
Methods: All medical records of the breast cancer patients taking LTZ with or without ANZ between 2004 and 2013 were reviewed. All patients were divided into two groups: the LTZ group included patients treated with LTZ alone, and the A-LTZ group included patients treated with ANZ who were automatically changed to LTZ due to change of the reimbursement policy.
Results: From 359 cases, there were 179 cases in the LTZ group and 180 cases in the A-LTZ group. The mean age of patients in the LTZ group was 53.7 years and in the A-LTZ group was 54.2 years. The distribution of clinical stages among the LTZ group versus the A-LTZ group was 21 versus 4 (stage 1), 86 versus 116 (stage 2), 55 versus 46 (stage 3), and 17 versus 14 (stage 4), respectively. Among the LTZ patients, 63.7% took aromatase inhibitor monotherapy and 36.3% had a switching strategy, while in the A-LTZ group, 53.9% took AI monotherapy and 46.1% had a switching strategy. OS of the A-LTZ group was longer than that of the LTZ group.
Conclusion: The patients in A-LTZ, taking ANZ followed by LTZ had better OS than those in LTZ, taking LTZ alone.
Keywords: estrogen receptor-positive; hormonal responsive; tamoxifen.
Figures
References
-
- Connor C, Attai D. Adjuvant endocrine therapy for the surgeon: options, side effects, and their management. Ann Surg Oncol. 2013;20(10):3188–3193. - PubMed
-
- Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. New Engl J Med. 2003;348(24):2431–2442. - PubMed
-
- Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M, Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9(1):45–53. - PubMed
-
- Cuzick J, Sestak I, Baum M, et al. ATAC/LATTE Investigators Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135–1141. - PubMed
-
- Davies C, Pan H, Godwin J, et al. Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. - PMC - PubMed
LinkOut - more resources
Full Text Sources

