Gastrointestinal injury associated with NSAID use: a case study and review of risk factors and preventative strategies
- PMID: 25653559
- PMCID: PMC4310346
- DOI: 10.2147/DHPS.S71976
Gastrointestinal injury associated with NSAID use: a case study and review of risk factors and preventative strategies
Abstract
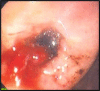
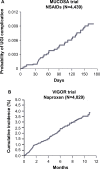
Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective anti-inflammatory and analgesic agents and are among the most commonly used classes of medications worldwide. However, their use has been associated with potentially serious dose-dependent gastrointestinal (GI) complications such as upper GI bleeding. GI complications resulting from NSAID use are among the most common drug side effects in the United States, due to the widespread use of NSAIDs. The risk of upper GI complications can occur even with short-term NSAID use, and the rate of events is linear over time with continued use. Although gastroprotective therapies are available, they are underused, and patient and physician awareness and recognition of some of the factors influencing the development of NSAID-related upper GI complications are limited. Herein, we present a case report of a patient experiencing a gastric ulcer following NSAID use and examine some of the risk factors and potential strategies for prevention of upper GI mucosal injuries and associated bleeding following NSAID use. These risk factors include advanced age, previous history of GI injury, and concurrent use of medications such as anticoagulants, aspirin, corticosteroids, and selective serotonin reuptake inhibitors. Strategies for prevention of GI injuries include anti-secretory agents, gastroprotective agents, alternative NSAID formulations, and nonpharmacologic therapies. Greater awareness of the risk factors and potential therapies for GI complications resulting from NSAID use could help improve outcomes for patients requiring NSAID treatment.
Keywords: GI bleed; NSAID; gastrointestinal; side effects; ulcer.
Figures
References
-
- Wilcox CM, Cryer B, Triadafilopoulos G. Patterns of use and public perception of over-the-counter pain relievers: focus on nonsteroidal antiinflammatory drugs. J Rheumatol. 2005;32(11):2218–2224. - PubMed
-
- Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol Drug Saf. 2014;23(1):43–50. - PubMed
-
- Goldstein JL, Lefkowith JB. Public misunderstanding of nonsteroidal antiinflammatory drug (NSAID)-mediated gastrointestinal (GI) toxicity: a serious potential health threat. Gastroenterology. 1998;114:A136.
-
- Capone ML, Tacconelli S, Rodriguez LG, Patrignani P. NSAIDs and cardiovascular disease: transducing human pharmacology results into clinical read-outs in the general population. Pharmacol Rep. 2010;62(3):530–535. - PubMed
-
- García Rodríguez LA, Tacconelli S, Patrignani P. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J Am Coll Cardiol. 2008;52(20):1628–1636. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

