Gene expression changes in spinal motoneurons of the SOD1(G93A) transgenic model for ALS after treatment with G-CSF
- PMID: 25653590
- PMCID: PMC4299451
- DOI: 10.3389/fncel.2014.00464
Gene expression changes in spinal motoneurons of the SOD1(G93A) transgenic model for ALS after treatment with G-CSF
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is an incurable fatal motoneuron disease with a lifetime risk of approximately 1:400. It is characterized by progressive weakness, muscle wasting, and death ensuing 3-5 years after diagnosis. Granulocyte-colony stimulating factor (G-CSF) is a drug candidate for ALS, with evidence for efficacy from animal studies and interesting data from pilot clinical trials. To gain insight into the disease mechanisms and mode of action of G-CSF, we performed gene expression profiling on isolated lumbar motoneurons from SOD1(G93A) mice, the most frequently studied animal model for ALS, with and without G-CSF treatment.
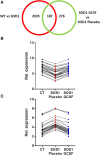
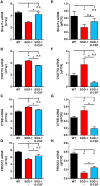
Results: Motoneurons from SOD1(G93A) mice present a distinct gene expression profile in comparison to controls already at an early disease stage (11 weeks of age), when treatment was initiated. The degree of deregulation increases at a time where motor symptoms are obvious (15 weeks of age). Upon G-CSF treatment, transcriptomic deregulations of SOD1(G93A) motoneurons were notably restored. Discriminant analysis revealed that SOD1 mice treated with G-CSF has a transcriptom close to presymptomatic SOD1 mice or wild type mice. Some interesting genes modulated by G-CSF treatment relate to neuromuscular function such as CCR4-NOT or Prss12.
Conclusions: Our data suggest that G-CSF is able to re-adjust gene expression in symptomatic SOD1(G93A) motoneurons. This provides further arguments for G-CSF as a promising drug candidate for ALS.
Keywords: ALS; G-CSF; gene expression; laser microdissection; motoneuron; mouse model; neurodegeneration.
Figures


References
-
- Averill S., Michael G. J., Shortland P. J., Leavesley R. C., King V. R., Bradbury E. J. (2004). NGF and GDNF ameliorate the increase in ATF3 expression which occurs in dorsal root ganglion cells in response to peripheral nerve injury. Eur. J. Neurosci. 19, 1437–1445. 10.1111/j.1460-9568.2004.03241.x - DOI - PubMed
-
- Cahoy J. D., Emery B., Kaushal A., Foo L. C., Zamanian J. L., Christopherson K. S., et al. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278. 10.1523/JNEUROSCI.4178-07.2008 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

