Spatial information in large-scale neural recordings
- PMID: 25653613
- PMCID: PMC4301009
- DOI: 10.3389/fncom.2014.00172
Spatial information in large-scale neural recordings
Abstract
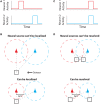
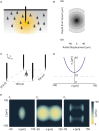
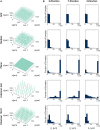
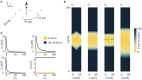
To record from a given neuron, a recording technology must be able to separate the activity of that neuron from the activity of its neighbors. Here, we develop a Fisher information based framework to determine the conditions under which this is feasible for a given technology. This framework combines measurable point spread functions with measurable noise distributions to produce theoretical bounds on the precision with which a recording technology can localize neural activities. If there is sufficient information to uniquely localize neural activities, then a technology will, from an information theoretic perspective, be able to record from these neurons. We (1) describe this framework, and (2) demonstrate its application in model experiments. This method generalizes to many recording devices that resolve objects in space and should be useful in the design of next-generation scalable neural recording systems.
Keywords: electrical recording; extracellular recording; fisher information; neural recording; optics; resolution; statistics; technology design.
Figures




References
-
- Airy G. B. (1835). On the diffraction of an object-glass with circular aperture. Trans. Cambridge Philos. Soc. 5:283.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

