Sphingomyelinase promotes oxidant production and skeletal muscle contractile dysfunction through activation of NADPH oxidase
- PMID: 25653619
- PMCID: PMC4300905
- DOI: 10.3389/fphys.2014.00530
Sphingomyelinase promotes oxidant production and skeletal muscle contractile dysfunction through activation of NADPH oxidase
Abstract
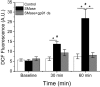
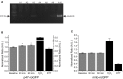
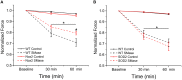
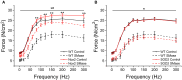
Elevated concentrations of sphingomyelinase (SMase) have been detected in a variety of diseases. SMase has been shown to increase muscle derived oxidants and decrease skeletal muscle force; however, the sub-cellular site of oxidant production has not been elucidated. Using redox sensitive biosensors targeted to the mitochondria and NADPH oxidase (Nox2), we demonstrate that SMase increased Nox2-dependent ROS and had no effect on mitochondrial ROS in isolated FDB fibers. Pharmacological inhibition and genetic knockdown of Nox2 activity prevented SMase induced ROS production and provided protection against decreased force production in the diaphragm. In contrast, genetic overexpression of superoxide dismutase within the mitochondria did not prevent increased ROS production and offered no protection against decreased diaphragm function in response to SMase. Our study shows that SMase induced ROS production occurs in specific sub-cellular regions of skeletal muscle; however, the increased ROS does not completely account for the decrease in muscle function.
Keywords: Nox2; ROS; force; skeletal muscle; sphingomyelinase.
Figures


Similar articles
-
Diaphragm dysfunction caused by sphingomyelinase requires the p47(phox) subunit of NADPH oxidase.Respir Physiol Neurobiol. 2015 Jan 1;205:47-52. doi: 10.1016/j.resp.2014.10.011. Epub 2014 Oct 24. Respir Physiol Neurobiol. 2015. PMID: 25448394 Free PMC article.
-
Sphingomyelinase depresses force and calcium sensitivity of the contractile apparatus in mouse diaphragm muscle fibers.J Appl Physiol (1985). 2012 May;112(9):1538-45. doi: 10.1152/japplphysiol.01269.2011. Epub 2012 Feb 23. J Appl Physiol (1985). 2012. PMID: 22362402 Free PMC article.
-
Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue.Am J Physiol Cell Physiol. 2010 Sep;299(3):C552-60. doi: 10.1152/ajpcell.00065.2010. Epub 2010 Jun 2. Am J Physiol Cell Physiol. 2010. PMID: 20519448 Free PMC article.
-
The Emerging Roles of Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2 in Skeletal Muscle Redox Signaling and Metabolism.Antioxid Redox Signal. 2019 Dec 20;31(18):1371-1410. doi: 10.1089/ars.2018.7678. Epub 2019 Nov 1. Antioxid Redox Signal. 2019. PMID: 31588777 Free PMC article. Review.
-
Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species.Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):897-906. doi: 10.1016/j.bbabio.2010.01.032. Epub 2010 Feb 1. Biochim Biophys Acta. 2010. PMID: 20122895 Review.
Cited by
-
Skeletal muscle redox signaling in rheumatoid arthritis.Clin Sci (Lond). 2020 Nov 13;134(21):2835-2850. doi: 10.1042/CS20190728. Clin Sci (Lond). 2020. PMID: 33146370 Free PMC article. Review.
-
Small-hairpin RNA and pharmacological targeting of neutral sphingomyelinase prevent diaphragm weakness in rats with heart failure and reduced ejection fraction.Am J Physiol Lung Cell Mol Physiol. 2019 Apr 1;316(4):L679-L690. doi: 10.1152/ajplung.00516.2018. Epub 2019 Jan 31. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30702345 Free PMC article.
-
Muscle Weakness in Rheumatoid Arthritis: The Role of Ca2+ and Free Radical Signaling.EBioMedicine. 2017 Sep;23:12-19. doi: 10.1016/j.ebiom.2017.07.023. Epub 2017 Jul 26. EBioMedicine. 2017. PMID: 28781131 Free PMC article. Review.
-
Diaphragm abnormalities in heart failure and aging: mechanisms and integration of cardiovascular and respiratory pathophysiology.Heart Fail Rev. 2017 Mar;22(2):191-207. doi: 10.1007/s10741-016-9549-4. Heart Fail Rev. 2017. PMID: 27000754 Free PMC article. Review.
-
Sphingomyelinase activity promotes atrophy and attenuates force in human muscle fibres and is elevated in heart failure patients.J Cachexia Sarcopenia Muscle. 2022 Oct;13(5):2551-2561. doi: 10.1002/jcsm.13029. Epub 2022 Jul 18. J Cachexia Sarcopenia Muscle. 2022. PMID: 35852046 Free PMC article.
References
-
- Bruton J. D., Place N., Yamada T., Silva J. P., Andrade F. H., Dahlstedt A. J., et al. . (2008). Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J. Physiol. 586, 175–184. 10.1113/jphysiol.2007.147470 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

