Sweet taste receptor signaling network: possible implication for cognitive functioning
- PMID: 25653876
- PMCID: PMC4306214
- DOI: 10.1155/2015/606479
Sweet taste receptor signaling network: possible implication for cognitive functioning
Abstract
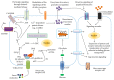
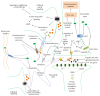
Sweet taste receptors are transmembrane protein network specialized in the transmission of information from special "sweet" molecules into the intracellular domain. These receptors can sense the taste of a range of molecules and transmit the information downstream to several acceptors, modulate cell specific functions and metabolism, and mediate cell-to-cell coupling through paracrine mechanism. Recent reports indicate that sweet taste receptors are widely distributed in the body and serves specific function relative to their localization. Due to their pleiotropic signaling properties and multisubstrate ligand affinity, sweet taste receptors are able to cooperatively bind multiple substances and mediate signaling by other receptors. Based on increasing evidence about the role of these receptors in the initiation and control of absorption and metabolism, and the pivotal role of metabolic (glucose) regulation in the central nervous system functioning, we propose a possible implication of sweet taste receptor signaling in modulating cognitive functioning.
Figures
Similar articles
-
Chloride ions evoke taste sensations by binding to the extracellular ligand-binding domain of sweet/umami taste receptors.Elife. 2023 Feb 28;12:e84291. doi: 10.7554/eLife.84291. Elife. 2023. PMID: 36852482 Free PMC article.
-
An alternative pathway for sweet sensation: possible mechanisms and physiological relevance.Pflugers Arch. 2020 Dec;472(12):1667-1691. doi: 10.1007/s00424-020-02467-1. Epub 2020 Oct 8. Pflugers Arch. 2020. PMID: 33030576 Review.
-
Whole-Brain Mapping of the Expression Pattern of T1R2, a Subunit Specific to the Sweet Taste Receptor.Front Neuroanat. 2021 Oct 28;15:751839. doi: 10.3389/fnana.2021.751839. eCollection 2021. Front Neuroanat. 2021. PMID: 34776881 Free PMC article.
-
The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY).Clin Nutr. 2011 Aug;30(4):524-32. doi: 10.1016/j.clnu.2011.01.007. Epub 2011 Feb 15. Clin Nutr. 2011. PMID: 21324568
-
Sweet Taste Signaling: The Core Pathways and Regulatory Mechanisms.Int J Mol Sci. 2022 Jul 26;23(15):8225. doi: 10.3390/ijms23158225. Int J Mol Sci. 2022. PMID: 35897802 Free PMC article. Review.
Cited by
-
Effect of 100% Orange Juice and a Volume-Matched Sugar-Sweetened Drink on Subjective Appetite, Food Intake, and Glycemic Response in Adults.Nutrients. 2024 Jan 12;16(2):242. doi: 10.3390/nu16020242. Nutrients. 2024. PMID: 38257135 Free PMC article.
-
Stomach 'tastes' the food and adjusts its emptying: A neurophysiological hypothesis (Review).Exp Ther Med. 2020 Sep;20(3):2392-2395. doi: 10.3892/etm.2020.8874. Epub 2020 Jun 11. Exp Ther Med. 2020. PMID: 32765721 Free PMC article. Review.
-
Determinants of Sweetness Preference: A Scoping Review of Human Studies.Nutrients. 2020 Mar 8;12(3):718. doi: 10.3390/nu12030718. Nutrients. 2020. PMID: 32182697 Free PMC article.
-
Effect of Commercially Available Sugar-Sweetened Beverages on Subjective Appetite and Short-Term Food Intake in Boys.Nutrients. 2019 Jan 26;11(2):270. doi: 10.3390/nu11020270. Nutrients. 2019. PMID: 30691085 Free PMC article. Clinical Trial.
-
Brain Glucose-Sensing Mechanism and Energy Homeostasis.Mol Neurobiol. 2019 Feb;56(2):769-796. doi: 10.1007/s12035-018-1099-4. Epub 2018 May 24. Mol Neurobiol. 2019. PMID: 29796992 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

