The lectin pathway of complement and rheumatic heart disease
- PMID: 25654073
- PMCID: PMC4300866
- DOI: 10.3389/fped.2014.00148
The lectin pathway of complement and rheumatic heart disease
Abstract
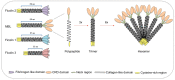
The innate immune system is the first line of host defense against infection and is comprised of humoral and cellular mechanisms that recognize potential pathogens within minutes or hours of entry. The effector components of innate immunity include epithelial barriers, phagocytes, and natural killer cells, as well as cytokines and the complement system. Complement plays an important role in the immediate response against microorganisms, including Streptococcus sp. The lectin pathway is one of three pathways by which the complement system can be activated. This pathway is initiated by the binding of mannose-binding lectin (MBL), collectin 11 (CL-K1), and ficolins (Ficolin-1, Ficolin-2, and Ficolin-3) to microbial surface oligosaccharides and acetylated residues, respectively. Upon binding to target molecules, MBL, CL-K1, and ficolins form complexes with MBL-associated serine proteases 1 and 2 (MASP-1 and MASP-2), which cleave C4 and C2 forming the C3 convertase (C4b2a). Subsequent activation of complement cascade leads to opsonization, phagocytosis, and lysis of target microorganisms through the formation of the membrane-attack complex. In addition, activation of complement may induce several inflammatory effects, such as expression of adhesion molecules, chemotaxis and activation of leukocytes, release of reactive oxygen species, and secretion of cytokines and chemokines. In this chapter, we review the general aspects of the structure, function, and genetic polymorphism of lectin-pathway components and discuss most recent understanding on the role of the lectin pathway in the predisposition and clinical progression of Rheumatic Fever.
Keywords: MBL; complement system; ficolins; gene polymorphisms; lectin pathway.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

