Bridging the gap: a roadmap to breaking the biological design barrier
- PMID: 25654077
- PMCID: PMC4299508
- DOI: 10.3389/fbioe.2014.00087
Bridging the gap: a roadmap to breaking the biological design barrier
Abstract
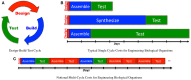
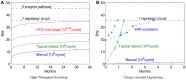
This paper presents an analysis of an emerging bottleneck in organism engineering, and paths by which it may be overcome. Recent years have seen the development of a profusion of synthetic biology tools, largely falling into two categories: high-level "design" tools aimed at mapping from organism specifications to nucleic acid sequences implementing those specifications, and low-level "build and test" tools aimed at faster, cheaper, and more reliable fabrication of those sequences and assays of their behavior in engineered biological organisms. Between the two families, however, there is a major gap: we still largely lack the predictive models and component characterization data required to effectively determine which of the many possible candidate sequences considered in the design phase are the most likely to produce useful results when built and tested. As low-level tools continue to mature, the bottleneck in biological systems engineering is shifting to be dominated by design, making this gap a critical barrier to progress. Considering how to address this gap, we find that widespread adoption of readily available analytic and assay methods is likely to lead to rapid improvement in available predictive models and component characterization models, as evidenced by a number of recent results. Such an enabling development is, in turn, likely to allow high-level tools to break the design barrier and support rapid development of transformative biological applications.
Keywords: automation; calibrated flow cytometry; design; metrology; organism engineering; prediction; synthetic biology.
Figures





References
-
- Adler A., Yaman F., Beal J. (2014). TASBE Tools. Available at: https://synbiotools.bbn.com/
-
- Amdahl G. M. (1967). “Validity of the single processor approach to achieving large-scale computing capabilities,” in AFIPS Conference Proceedings, Vol. 30 (Reston, VA: AFIPS Press), 483–485.
-
- Beal J., Bachrach J. (2008). “Cells are plausible targets for high-level spatial languages,” in Proceedings of the 2008 Second IEEE International Conference on Self-Adaptive and Self-Organizing Systems Workshops (SASOW’08) (Washington, DC: IEEE Computer Society), 284–291.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

