Systems and photosystems: cellular limits of autotrophic productivity in cyanobacteria
- PMID: 25654078
- PMCID: PMC4299538
- DOI: 10.3389/fbioe.2015.00001
Systems and photosystems: cellular limits of autotrophic productivity in cyanobacteria
Abstract
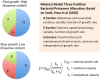
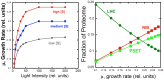
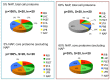
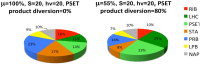
Recent advances in the modeling of microbial growth and metabolism have shown that growth rate critically depends upon the optimal allocation of finite proteomic resources among different cellular functions and that modeling growth rates becomes more realistic with the explicit accounting for the costs of macromolecular synthesis, most importantly, protein expression. The "proteomic constraint" is considered together with its application to understanding photosynthetic microbial growth. The central hypothesis is that physical limits of cellular space (and corresponding solvation capacity) in conjunction with cell surface-to-volume ratios represent the underlying constraints on the maximal rate of autotrophic microbial growth. The limitation of cellular space thus constrains the size the total complement of macromolecules, dissolved ions, and metabolites. To a first approximation, the upper limit in the cellular amount of the total proteome is bounded this space limit. This predicts that adaptation to osmotic stress will result in lower maximal growth rates due to decreased cellular concentrations of core metabolic proteins necessary for cell growth owing the accumulation of compatible osmolytes, as surmised previously. The finite capacity of membrane and cytoplasmic space also leads to the hypothesis that the species-specific differences in maximal growth rates likely reflect differences in the allocation of space to niche-specific proteins with the corresponding diminution of space devoted to other functions including proteins of core autotrophic metabolism, which drive cell reproduction. An optimization model for autotrophic microbial growth, the autotrophic replicator model, was developed based upon previous work investigating heterotrophic growth. The present model describes autotrophic growth in terms of the allocation protein resources among core functional groups including the photosynthetic electron transport chain, light-harvesting antennae, and the ribosome groups.
Keywords: cyanobacteria; growth rate; molecular crowding; optimization; photosynthesis; ribosomes.
Figures


References
-
- Andrei N. (2013). Nonlinear Optimization Applications Using the GAMS Technology. New York: Springer.
-
- Atkinson D. E. (1977). Cellular Energy Metabolism and its Regulation. New York: Academic Press.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

