Regulation of ICAM-1 in cells of the monocyte/macrophage system in microgravity
- PMID: 25654110
- PMCID: PMC4309248
- DOI: 10.1155/2015/538786
Regulation of ICAM-1 in cells of the monocyte/macrophage system in microgravity
Abstract
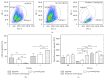
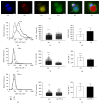
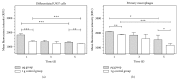
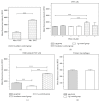
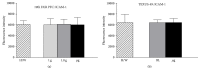
Cells of the immune system are highly sensitive to altered gravity, and the monocyte as well as the macrophage function is proven to be impaired under microgravity conditions. In our study, we investigated the surface expression of ICAM-1 protein and expression of ICAM-1 mRNA in cells of the monocyte/macrophage system in microgravity during clinostat, parabolic flight, sounding rocket, and orbital experiments. In murine BV-2 microglial cells, we detected a downregulation of ICAM-1 expression in clinorotation experiments and a rapid and reversible downregulation in the microgravity phase of parabolic flight experiments. In contrast, ICAM-1 expression increased in macrophage-like differentiated human U937 cells during the microgravity phase of parabolic flights and in long-term microgravity provided by a 2D clinostat or during the orbital SIMBOX/Shenzhou-8 mission. In nondifferentiated U937 cells, no effect of microgravity on ICAM-1 expression could be observed during parabolic flight experiments. We conclude that disturbed immune function in microgravity could be a consequence of ICAM-1 modulation in the monocyte/macrophage system, which in turn could have a strong impact on the interaction with T lymphocytes and cell migration. Thus, ICAM-1 can be considered as a rapid-reacting and sustained gravity-regulated molecule in mammalian cells.
Figures


References
-
- Ullrich O., Thiel C. S. Gravitational force: triggered stress in cells of the immune system. In: Choukèr A., editor. Stress Challenges, and Immunity in Space: From Mechanisms to Monitoring and Preventive Strategies. chapter 14. Berlin, Germany: Springer; 2012. pp. 187–202. - DOI
-
- Konstantinova I. V., Antropova E. N., Legen'kov V. I., Zazhireĭ V. D. Reactivity of lymphoid blood cells in the crew of “Soiuz-6”, “Soiuz-7” and “Soiuz-8” spacecraft before and after flight. Kosmicheskaia Biologiia i Aviakosmicheskaia Meditsina. 1973;7(6):35–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

