HIV competition dynamics over sexual networks: first comer advantage conserves founder effects
- PMID: 25654450
- PMCID: PMC4318579
- DOI: 10.1371/journal.pcbi.1004093
HIV competition dynamics over sexual networks: first comer advantage conserves founder effects
Abstract
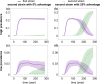
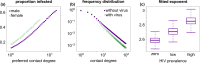
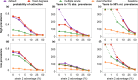
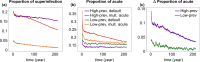
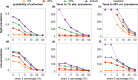
Outside Africa, the global phylogeography of HIV is characterized by compartmentalized local epidemics that are typically dominated by a single subtype, which indicates strong founder effects. We hypothesized that the competition of viral strains at the epidemic level may involve an advantage of the resident strain that was the first to colonize a population. Such an effect would slow down the invasion of new strains, and thus also the diversification of the epidemic. We developed a stochastic modelling framework to simulate HIV epidemics over dynamic contact networks. We simulated epidemics in which the second strain was introduced into a population where the first strain had established a steady-state epidemic, and assessed whether, and on what time scale, the second strain was able to spread in the population. Simulations were parameterized based on empirical data; we tested scenarios with varying levels of overall prevalence. The spread of the second strain occurred on a much slower time scale compared with the initial expansion of the first strain. With strains of equal transmission efficiency, the second strain was unable to invade on a time scale relevant for the history of the HIV pandemic. To become dominant over a time scale of decades, the second strain needed considerable (>25%) advantage in transmission efficiency over the resident strain. The inhibition effect was weaker if the second strain was introduced while the first strain was still in its growth phase. We also tested how possible mechanisms of interference (inhibition of superinfection, depletion of highly connected hubs in the network, one-time acute peak of infectiousness) contribute to the inhibition effect. Our simulations confirmed a strong first comer advantage in the competition dynamics of HIV at the population level, which may explain the global phylogeography of the virus and may influence the future evolution of the pandemic.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Korber B, Muldoon M, Theiler J, Gao F, Gupta R, et al. (2000) Timing the ancestor of the HIV-1 pandemic strains. Science 288: 1789–1796. - PubMed
-
- Rambaut A, Robertson DL, Pybus OG, Peeters M, Holmes EC (2001) Human immunodeficiency virus. Phylogeny and the origin of HIV-1. Nature 410: 1047–1048. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

