Brucella CβG induces a dual pro- and anti-inflammatory response leading to a transient neutrophil recruitment
- PMID: 25654761
- PMCID: PMC4603436
- DOI: 10.4161/21505594.2014.979692
Brucella CβG induces a dual pro- and anti-inflammatory response leading to a transient neutrophil recruitment
Abstract
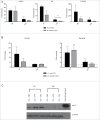
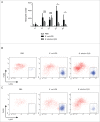
Brucella is the causing agent of a chronic zoonosis called brucellosis. The Brucella β-1,2 cyclic glucan (CβG) is a virulence factor, which has been described as a potent immune stimulator, albeit with no toxicity for cells and animals. We first used a genome-wide approach to characterize human myeloid dendritic cell (mDC) responses to CβG. Transcripts related to inflammation (IL-6, IL2RA, PTGS2), chemokine (CXCR7, CXCL2) and anti-inflammatory pathways (TNFAIP6, SOCS3) were highly expressed in CβG-treated mDC. In mouse GMCSF-derived DC, CβG triggered the expression of both activation (CXCL2, KC) and inhibition (SOCS3 and TNFAIP6) molecules. We then characterized the inflammatory infiltrates at the level of mouse ear when injected with CβG or LPS. CβG yielded a lower and transient recruitment of neutrophils compared to LPS. The consequence of these dual pro- and anti-inflammatory signals triggered by CβG corresponds to the induction of a controlled local inflammation.
Keywords: Brucella; CβG, Beta-1,2 cyclic glucan; DC, dendritic cells; E. coli, Escherichia coli; LPS, lipopolysaccharide; cyclic β glucan; inflammation; lipopolysaccharide; neutrophil.
Figures


References
-
- Ian Maudlin SW-M. The Control of Neglected Zoonotic Diseases – A Route to Poverty Alleviation. Geneva: WHO, 2006.
-
- de Jong MF, Tsolis RM. Brucellosis and type IV secretion. Future Microbiol 2012; 7:47-58; PMID:22191446; http://dx.doi.org/10.2217/fmb.11.136 - DOI - PubMed
-
- Lacerda TL, Salcedo SP, Gorvel JP. Brucella T4SS: the VIP pass inside host cells. Curr Opin Microbiol 2013; 16:45-51; PMID:23318140; http://dx.doi.org/10.1016/j.mib.2012.11.005 - DOI - PubMed
-
- Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, Muller A, Lapaque N, Demaria O, Alexopoulou L, et al. . Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathogens 2008; 4:e21; PMID:18266466; http://dx.doi.org/10.1371/journal.ppat.0040021 - DOI - PMC - PubMed
-
- Salcedo SP, Marchesini MI, Degos C, Terwagne M, Von Bargen K, Lepidi H, Herrmann CK, Santos Lacerda TL, Imbert PR, Pierre P, et al. . BtpB, a novel Brucella TIR-containing effector protein with immune modulatory functions. Front Cell Infect Microbiol 2013; 3:28; PMID:23847770; http://dx.doi.org/10.3389/fcimb.2013.00028 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
