TSC2/mTORC1 signaling controls Paneth and goblet cell differentiation in the intestinal epithelium
- PMID: 25654764
- PMCID: PMC4669793
- DOI: 10.1038/cddis.2014.588
TSC2/mTORC1 signaling controls Paneth and goblet cell differentiation in the intestinal epithelium
Abstract
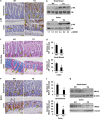
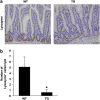
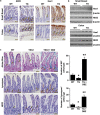
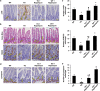
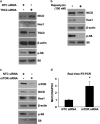
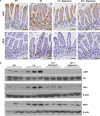
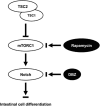
The intestinal mucosa undergoes a continual process of proliferation, differentiation and apoptosis, which is regulated by multiple signaling pathways. Notch signaling is critical for the control of intestinal stem cell maintenance and differentiation. However, the precise mechanisms involved in the regulation of differentiation are not fully understood. Previously, we have shown that tuberous sclerosis 2 (TSC2) positively regulates the expression of the goblet cell differentiation marker, MUC2, in intestinal cells. Using transgenic mice constitutively expressing a dominant negative TSC2 allele, we observed that TSC2 inactivation increased mTORC1 and Notch activities, and altered differentiation throughout the intestinal epithelium, with a marked decrease in the goblet and Paneth cell lineages. Conversely, treatment of mice with either Notch inhibitor dibenzazepine (DBZ) or mTORC1 inhibitor rapamycin significantly attenuated the reduction of goblet and Paneth cells. Accordingly, knockdown of TSC2 activated, whereas knockdown of mTOR or treatment with rapamycin decreased, the activity of Notch signaling in the intestinal cell line LS174T. Importantly, our findings demonstrate that TSC2/mTORC1 signaling contributes to the maintenance of intestinal epithelium homeostasis by regulating Notch activity.
Figures
Similar articles
-
Numb modulates intestinal epithelial cells toward goblet cell phenotype by inhibiting the Notch signaling pathway.Exp Cell Res. 2011 Jul 1;317(11):1640-8. doi: 10.1016/j.yexcr.2011.04.008. Epub 2011 Apr 30. Exp Cell Res. 2011. PMID: 21557937
-
Requirement of Notch activation during regeneration of the intestinal epithelia.Am J Physiol Gastrointest Liver Physiol. 2009 Jan;296(1):G23-35. doi: 10.1152/ajpgi.90225.2008. Epub 2008 Nov 20. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19023031
-
Requirement of Math1 for secretory cell lineage commitment in the mouse intestine.Science. 2001 Dec 7;294(5549):2155-8. doi: 10.1126/science.1065718. Science. 2001. PMID: 11739954
-
Paneth Cells: Dispensable yet Irreplaceable for the Intestinal Stem Cell Niche.Cell Mol Gastroenterol Hepatol. 2025;19(4):101443. doi: 10.1016/j.jcmgh.2024.101443. Epub 2024 Dec 19. Cell Mol Gastroenterol Hepatol. 2025. PMID: 39708920 Free PMC article. Review.
-
From intestinal stem cells to inflammatory bowel diseases.World J Gastroenterol. 2011 Jul 21;17(27):3198-203. doi: 10.3748/wjg.v17.i27.3198. World J Gastroenterol. 2011. PMID: 21912468 Free PMC article. Review.
Cited by
-
Cbl and Cbl-b ubiquitin ligases are essential for intestinal epithelial stem cell maintenance.iScience. 2024 May 8;27(6):109912. doi: 10.1016/j.isci.2024.109912. eCollection 2024 Jun 21. iScience. 2024. PMID: 38974465 Free PMC article.
-
Enrichment of in vivo transcription data from dietary intervention studies with in vitro data provides improved insight into gene regulation mechanisms in the intestinal mucosa.Genes Nutr. 2017 Apr 13;12:11. doi: 10.1186/s12263-017-0559-1. eCollection 2017. Genes Nutr. 2017. PMID: 28413565 Free PMC article.
-
Chronic rapamycin treatment on the nutrient utilization and metabolism of juvenile turbot (Psetta maxima).Sci Rep. 2016 Jun 16;6:28068. doi: 10.1038/srep28068. Sci Rep. 2016. PMID: 27305975 Free PMC article.
-
Role of mTORC1 in intestinal epithelial repair and tumorigenesis.Cell Mol Life Sci. 2019 Jul;76(13):2525-2546. doi: 10.1007/s00018-019-03085-6. Epub 2019 Apr 3. Cell Mol Life Sci. 2019. PMID: 30944973 Free PMC article. Review.
-
Building better barriers: how nutrition and undernutrition impact pediatric intestinal health.Front Immunol. 2023 Jul 21;14:1192936. doi: 10.3389/fimmu.2023.1192936. eCollection 2023. Front Immunol. 2023. PMID: 37545496 Free PMC article. Review.
References
-
- 3Gunther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut 2013; 62: 1062–1071. - PubMed
-
- 4Gersemann M, Wehkamp J, Stange EF. Innate immune dysfunction in inflammatory bowel disease. J Intern Med 2012; 271: 421–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

