Direct evaluation of myocardial viability and stem cell engraftment demonstrates salvage of the injured myocardium
- PMID: 25654979
- PMCID: PMC4380765
- DOI: 10.1161/CIRCRESAHA.116.304668
Direct evaluation of myocardial viability and stem cell engraftment demonstrates salvage of the injured myocardium
Abstract
Rationale: The mechanism of functional restoration by stem cell therapy remains poorly understood. Novel manganese-enhanced MRI and bioluminescence reporter gene imaging were applied to follow myocardial viability and cell engraftment, respectively. Human-placenta-derived amniotic mesenchymal stem cells (AMCs) demonstrate unique immunoregulatory and precardiac properties. In this study, the restorative effects of 3 AMC-derived subpopulations were examined in a murine myocardial injury model: (1) unselected AMCs, (2) ckit(+)AMCs, and (3) AMC-derived induced pluripotent stem cells (MiPSCs).
Objective: To determine the differential restorative effects of the AMC-derived subpopulations in the murine myocardial injury model using multimodality imaging.
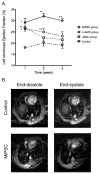
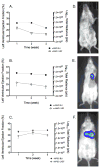
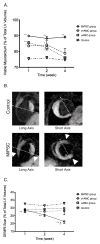
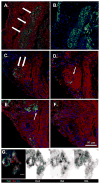
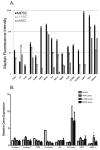
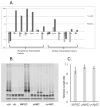
Methods and results: SCID (severe combined immunodeficiency) mice underwent left anterior descending artery ligation and were divided into 4 treatment arms: (1) normal saline control (n=14), (2) unselected AMCs (n=10), (3) ckit(+)AMCs (n=13), and (4) MiPSCs (n=11). Cardiac MRI assessed myocardial viability and left ventricular function, whereas bioluminescence imaging assessed stem cell engraftment during a 4-week period. Immunohistological labeling and reverse transcriptase polymerase chain reaction of the explanted myocardium were performed. The unselected AMC and ckit(+)AMC-treated mice demonstrated transient left ventricular functional improvement. However, the MiPSCs exhibited a significantly greater increase in left ventricular function compared with all the other groups during the entire 4-week period. Left ventricular functional improvement correlated with increased myocardial viability and sustained stem cell engraftment. The MiPSC-treated animals lacked any evidence of de novo cardiac differentiation.
Conclusion: The functional restoration seen in MiPSCs was characterized by increased myocardial viability and sustained engraftment without de novo cardiac differentiation, indicating salvage of the injured myocardium.
Keywords: magnetic resonance imaging; molecular imaging; multimodal imaging.
© 2015 American Heart Association, Inc.
Figures

References
-
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. - PubMed
-
- Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M, Ratajczak J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166–73. - PubMed
-
- Welt FG, Gallegos R, Connell J, Kajstura J, D’Amario D, Kwong RY, Coelho-Filho O, Shah R, Mitchell R, Leri A, Foley L, Anversa P, Pfeffer MA. Effect of cardiac stem cells on left-ventricular remodeling in a canine model of chronic myocardial infarction. Circ Heart Fail. 2013;6:99–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

