T-cell subsets in peripheral blood and tumors of patients treated with oncolytic adenoviruses
- PMID: 25655312
- PMCID: PMC4427873
- DOI: 10.1038/mt.2015.17
T-cell subsets in peripheral blood and tumors of patients treated with oncolytic adenoviruses
Erratum in
-
Corrigendum to "T-cell Subsets in Peripheral Blood and Tumors of Patients Treated With Oncolytic Adenoviruses".Mol Ther. 2016 Jun;24(6):1159. doi: 10.1038/mt.2016.65. Mol Ther. 2016. PMID: 27324446 Free PMC article. No abstract available.
Abstract
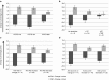
The quality of the antitumor immune response is decisive when developing new immunotherapies for cancer. Oncolytic adenoviruses cause a potent immunogenic stimulus and arming them with costimulatory molecules reshapes the immune response further. We evaluated peripheral blood T-cell subsets of 50 patients with refractory solid tumors undergoing treatment with oncolytic adenovirus. These data were compared to changes in antiviral and antitumor T cells, treatment efficacy, overall survival, and T-cell subsets in pre- and post-treatment tumor biopsies. Treatment caused a significant (P < 0.0001) shift in T-cell subsets in blood, characterized by a proportional increase of CD8(+) cells, and decrease of CD4(+) cells. Concomitant treatment with cyclophosphamide and temozolomide resulted in less CD4(+) decrease (P = 0.041) than cyclophosphamide only. Interestingly, we saw a correlation between T-cell changes in peripheral blood and the tumor site. This correlation was positive for CD8(+) and inverse for CD4(+) cells. These findings give insight to the interconnections between peripheral blood and tumor-infiltrating lymphocyte (TIL) populations regarding oncolytic virotherapy. In particular, our data suggest that induction of T-cell response is not sufficient for clinical response in the context of immunosuppressive tumors, and that peripheral blood T cells have a complicated and potentially misleading relationship with TILs.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

