Intraosseous delivery of lentiviral vectors targeting factor VIII expression in platelets corrects murine hemophilia A
- PMID: 25655313
- PMCID: PMC4395783
- DOI: 10.1038/mt.2015.20
Intraosseous delivery of lentiviral vectors targeting factor VIII expression in platelets corrects murine hemophilia A
Abstract
Intraosseous (IO) infusion of lentiviral vectors (LVs) for in situ gene transfer into bone marrow may avoid specific challenges posed by ex vivo gene delivery, including, in particular, the requirement of preconditioning. We utilized IO delivery of LVs encoding a GFP or factor VIII (FVIII) transgene directed by ubiquitous promoters (a MND or EF-1α-short element; M-GFP-LV, E-F8-LV) or a platelet-specific, glycoprotein-1bα promoter (G-GFP-LV, G-F8-LV). A single IO infusion of M-GFP-LV or G-GFP-LV achieved long-term and efficient GFP expression in Lineage(-)Sca1(+)c-Kit(+) hematopoietic stem cells and platelets, respectively. While E-F8-LV produced initially high-level FVIII expression, robust anti-FVIII immune responses eliminated functional FVIII in circulation. In contrast, IO delivery of G-F8-LV achieved long-term platelet-specific expression of FVIII, resulting in partial correction of hemophilia A. Furthermore, similar clinical benefit with G-F8-LV was achieved in animals with pre-existing anti-FVIII inhibitors. These findings further support platelets as an ideal FVIII delivery vehicle, as FVIII, stored in α-granules, is protected from neutralizing antibodies and, during bleeding, activated platelets locally excrete FVIII to promote clot formation. Overall, a single IO infusion of G-F8-LV was sufficient to correct hemophilia phenotype for long term, indicating that this approach may provide an effective means to permanently treat FVIII deficiency.
Figures
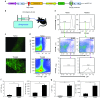



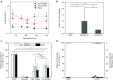

Comment in
-
A shot in the bone corrects a genetic disease.Mol Ther. 2015 Apr;23(4):614-5. doi: 10.1038/mt.2015.38. Mol Ther. 2015. PMID: 25849425 Free PMC article. No abstract available.
References
-
- Gringeri A, Mantovani LG, Scalone L, Mannucci PM. COCIS Study Group Cost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group. Blood. 2003;102:2358–2363. - PubMed
-
- Roth DA, Tawa NE, Jr, O'Brien JM, Treco DA, Selden RF. Factor VIII Transkaryotic Therapy Study Group Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med. 2001;344:1735–1742. - PubMed
-
- Powell JS, Ragni MV, White GC, 2nd, Lusher JM, Hillman-Wiseman C, Moon TE, et al. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood. 2003;102:2038–2045. - PubMed
-
- White GC., 2nd Gene therapy in hemophilia: clinical trials update. Thromb Haemost. 2001;86:172–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

