Recruitment of Donor T Cells to the Eyes During Ocular GVHD in Recipients of MHC-Matched Allogeneic Hematopoietic Stem Cell Transplants
- PMID: 25655798
- PMCID: PMC4406104
- DOI: 10.1167/iovs.14-15630
Recruitment of Donor T Cells to the Eyes During Ocular GVHD in Recipients of MHC-Matched Allogeneic Hematopoietic Stem Cell Transplants
Abstract
Purpose: The primary objective of the present study was to identify the kinetics and origin of ocular infiltrating T cells in a preclinical model of graft-versus-host disease (GVHD) that induces eye tissue damage.
Methods: Graft-versus-host disease was induced using an major histocompatibility complex (MHC)-matched, minor histocompatibility-mismatched hematopoietic stem cell transplant (HSCT) model. This approach, which utilized congenic and EGFP-labeled donor populations, mimics a matched, clinically unrelated donor (MUD) cell transplant. Systemic and ocular GVHD were assessed at varying time points using clinical examination, intravital microscopy, immune phenotype via flow cytometric analyses, and immunohistochemical staining.
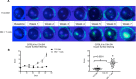
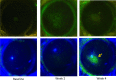
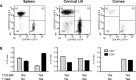
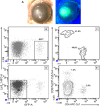
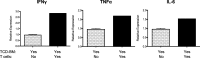
Results: Following transplant, we observed characteristic changes in GVHD-associated immune phenotype as well as clinical signs present in recipients post transplant. Notably, the kinetics of the systemic changes and the ocular damage paralleled what is observed clinically, including damage to the cornea as well as the conjunctiva and lacrimal gland. Importantly, the infiltrate contained predominantly donor CD4 as well as CD8 T cells with an activated phenotype and macrophages together with effector cytokines consistent with the presence of a TH1 alloreactive population.
Conclusions: Overall, the findings here unequivocally demonstrated that donor T cells compose part of the corneal and ocular adnexa infiltrate in animals undergoing ocular GVHD. In total, the results describe a novel and promising preclinical model characterized by both systemic and ocular changes as detected in significant numbers of patients undergoing GVHD following allo-HSCT, which can help facilitate dissecting the underlying immune mechanisms leading to damage associated with ocular GVHD.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

