Identification of p58IPK as a novel neuroprotective factor for retinal neurons
- PMID: 25655802
- PMCID: PMC4340432
- DOI: 10.1167/iovs.14-15196
Identification of p58IPK as a novel neuroprotective factor for retinal neurons
Abstract
Purpose: Endoplasmic reticulum (ER)-resident chaperone protein p58(IPK) plays a vital role in regulation of protein folding and biosynthesis. The goal of this study was to examine the role of p58(IPK) in retinal neuronal cells under normal and stressed conditions.
Methods: Retinal expression of p58(IPK), retinal morphology, apoptosis, ER stress, and apoptotic gene expression were examined in p58(IPK) knockout (KO) and/or wild-type (WT) mice with or without intravitreal injection of N-methyl-D-aspartic acid (NMDA). In in vitro experiments, differentiated R28 retinal neuronal cells transduced with adenovirus encoding p58(IPK) (Ad-p58(IPK)) or control virus (Ad-LacZ) were exposed to tunicamycin (TM) or hydrogen peroxide (H2O2). Levels of ER stress, apoptosis, and cell survival were evaluated.
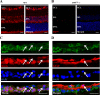
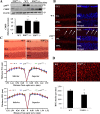
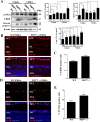
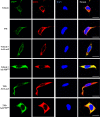
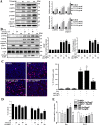
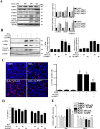
Results: Chaperone protein p58(IPK) is expressed predominantly in retinal ganglion cells (RGC), inner retinal neurons, and the photoreceptor inner segments. Mice lacking p58(IPK) exhibited increased CHOP expression and loss of RGCs with aging (8-10 months). Intravitreal injection of NMDA induced retinal ER stress and increased p58(IPK) expression in WT mice; this resulted in greater ER stress and enhanced RGC apoptosis in p58(IPK) KO mice. In cultured R28 cells, overexpression of p58(IPK) significantly reduced eIF2α phosphorylation, decreased CHOP expression, and alleviated the activation of caspase-3 and PARP. Overexpression of p58(IPK) also protected against oxidative and ER stress-induced cell apoptosis. Furthermore, p58(IPK) downregulated the proapoptotic gene Bax and upregulated the antiapoptotic gene Bcl-2 expression in stressed R28 cells.
Conclusions: Our study has demonstrated a protective role of p58(IPK) in retinal neurons, which may act in part through a mechanism involving modulation of ER homeostasis and apoptosis, particularly under conditions of cellular stresses.
Keywords: chaperone; endoplasmic reticulum stress; neuronal cells; p58IPK.
Copyright 2015 The Association for Research in Vision and Ophthalmology, Inc.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

