A randomized controlled study evaluating safety and efficacy of leuprorelin acetate every-3-months depot for 2 versus 3 or more years with tamoxifen for 5 years as adjuvant treatment in premenopausal patients with endocrine-responsive breast cancer
- PMID: 25655898
- PMCID: PMC4839052
- DOI: 10.1007/s12282-015-0593-z
A randomized controlled study evaluating safety and efficacy of leuprorelin acetate every-3-months depot for 2 versus 3 or more years with tamoxifen for 5 years as adjuvant treatment in premenopausal patients with endocrine-responsive breast cancer
Abstract
Background: Luteinizing hormone-releasing hormone (LH-RH) agonists provide effective adjuvant treatment for premenopausal women with endocrine-responsive breast cancer. Here, we investigated appropriate treatment durations of an LH-RH agonist, leuprorelin.
Methods: We conducted an open-label, randomized controlled pilot study to evaluate the safety and efficacy of leuprorelin subcutaneously administered every-3-months for 2 versus 3 or more, up to 5 years, together with daily tamoxifen for 5 years in premenopausal endocrine-responsive breast cancer patients. Primary endpoints were disease-free survival (DFS) and safety.
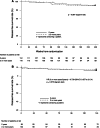
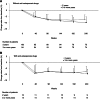
Results: Eligible patients (N = 222) were randomly assigned to receive leuprorelin for either 2 years (N = 112) or 3 or more years (N = 110) with tamoxifen for 5 years after surgery. Leuprorelin treatment for 3 or more years provided no significant difference in DFS rate over 2 years: 94.1 versus 91.8 % at 144 weeks (3 years) after the second year (week 96) and 90.8 versus 90.4 % at the fifth year (week 240). The overall survival rate was 100 % for both groups during the third through fifth year study period. There were no significant differences in the incidence of adverse events (AEs) between the 2 groups: most AEs were rated grade 1 or 2.
Conclusions: Adjuvant leuprorelin treatment for 3 or more years with tamoxifen showed a survival benefit and safety profile similar to that for 2 years in premenopausal endocrine-responsive breast cancer patients. No new safety signal was identified for long-term leuprorelin treatment. Longer follow-up observation is needed to determine the optimal duration of leuprorelin treatment.
Keywords: Adjuvant endocrine therapy; Disease-free survival (DFS); Leuprorelin acetate; Premenopausal endocrine-responsive breast cancer; Safety.
Figures


Similar articles
-
Efficacy and safety of leuprorelin acetate 6-month depot, TAP-144-SR (6M), in combination with tamoxifen in postoperative, premenopausal patients with hormone receptor-positive breast cancer: a phase III, randomized, open-label, parallel-group comparative study.Breast Cancer. 2017 Jan;24(1):161-170. doi: 10.1007/s12282-016-0691-6. Epub 2016 Mar 26. Breast Cancer. 2017. PMID: 27017207 Free PMC article. Clinical Trial.
-
A follow-up study of a randomized controlled study evaluating safety and efficacy of leuprorelin acetate every-3-month depot for 2 versus 3 or more years with tamoxifen for 5 years as adjuvant treatment in premenopausal patients with endocrine-responsive breast cancer.Breast Cancer. 2021 May;28(3):684-697. doi: 10.1007/s12282-020-01205-w. Epub 2021 Feb 27. Breast Cancer. 2021. PMID: 33638810 Free PMC article. Clinical Trial.
-
Comparison of quality of life between 2-year and 3-or-more-year administration of leuprorelin acetate every-3-months depot in combination with tamoxifen as adjuvant endocrine treatment in premenopausal patients with endocrine-responsive breast cancer: a randomized controlled trial.Support Care Cancer. 2018 Mar;26(3):933-945. doi: 10.1007/s00520-017-3914-2. Epub 2017 Oct 24. Support Care Cancer. 2018. PMID: 29063390 Free PMC article. Clinical Trial.
-
Luteinizing hormone-releasing hormone agonists in premenopausal hormone receptor-positive breast cancer.Clin Breast Cancer. 2007 Feb;7(6):455-64. doi: 10.3816/CBC.2007.n.002. Clin Breast Cancer. 2007. PMID: 17386122 Review.
-
New insights on the role of luteinizing hormone releasing hormone agonists in premenopausal early breast cancer patients.Cancer Treat Rev. 2016 Jan;42:18-23. doi: 10.1016/j.ctrv.2015.11.002. Epub 2015 Nov 21. Cancer Treat Rev. 2016. PMID: 26613834 Review.
Cited by
-
Tamoxifen Pharmacovigilance: Implications for Safe Use in the Future.Consult Pharm. 2017 Sep 1;32(9):535-546. doi: 10.4140/TCP.n.2017.535. Consult Pharm. 2017. PMID: 28855012 Free PMC article.
-
Efficacy and safety of leuprorelin acetate 6-month depot, TAP-144-SR (6M), in combination with tamoxifen in postoperative, premenopausal patients with hormone receptor-positive breast cancer: a phase III, randomized, open-label, parallel-group comparative study.Breast Cancer. 2017 Jan;24(1):161-170. doi: 10.1007/s12282-016-0691-6. Epub 2016 Mar 26. Breast Cancer. 2017. PMID: 27017207 Free PMC article. Clinical Trial.
-
A follow-up study of a randomized controlled study evaluating safety and efficacy of leuprorelin acetate every-3-month depot for 2 versus 3 or more years with tamoxifen for 5 years as adjuvant treatment in premenopausal patients with endocrine-responsive breast cancer.Breast Cancer. 2021 May;28(3):684-697. doi: 10.1007/s12282-020-01205-w. Epub 2021 Feb 27. Breast Cancer. 2021. PMID: 33638810 Free PMC article. Clinical Trial.
-
Therapeutic advances in hormone-dependent cancers: focus on prostate, breast and ovarian cancers.Endocr Connect. 2019 Feb 1;8(2):R10-R26. doi: 10.1530/EC-18-0425. Endocr Connect. 2019. PMID: 30640710 Free PMC article. Review.
-
An Overview of Long-Acting GnRH Agonists in Premenopausal Breast Cancer Patients: Survivorship Challenges and Management.Curr Oncol. 2024 Jul 25;31(8):4209-4224. doi: 10.3390/curroncol31080314. Curr Oncol. 2024. PMID: 39195297 Free PMC article. Review.
References
-
- Goldhirsch A,Glick JH, Gelber D, Coates AS, Senn HJ. Meeting highlights: International Consensus Panel on the treatment of primary breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin Oncol. 2001; 19:3817–3827. - PubMed
-
- Jonat W, Kaufmann M, Sauerbrei W, Blamey R, Cuzick J, Namer M, et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: the Zoladex Early Breast Cancer Research Association Study. J Clin Oncol. 2002;20:4628–4635. doi: 10.1200/JCO.2002.05.042. - DOI - PubMed
-
- International Breast Cancer Study Group (IBCSG) Castiglione-Gertsch M, O’Neill A, Price KN, Goldhirsch A, Coates AS, et al. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node–negative breast cancer: a randomized trial. J Natl Cancer Inst. 2003;95:1833–1846. doi: 10.1093/jnci/djg119. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

