Natural Green coating inhibits adhesion of clinically important bacteria
- PMID: 25655943
- PMCID: PMC4319173
- DOI: 10.1038/srep08287
Natural Green coating inhibits adhesion of clinically important bacteria
Abstract
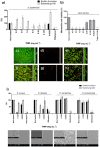
Despite many advances, biomaterial-associated infections continue to be a major clinical problem. In order to minimize bacterial adhesion, material surface modifications are currently being investigated and natural products possess large potential for the design of innovative surface coatings. We report the bioguided phytochemical investigation of Pityrocarpa moniliformis and the characterization of tannins by mass spectrometry. It was demonstrated that B-type linked proanthocyanidins-coated surfaces, here termed Green coatings, reduced Gram-positive bacterial adhesion and supported mammalian cell spreading. The proposed mechanism of bacterial attachment inhibition is based on electrostatic repulsion, high hydrophilicity and the steric hindrance provided by the coating that blocks bacterium-substratum interactions. This work shows the applicability of a prototype Green-coated surface that aims to promote necessary mammalian tissue compatibility, while reducing bacterial colonization.
Figures





References
-
- Bazaka K., Jacob M. V., Crawford R. J. & Ivanova E. P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 95, 299–311 (2012). - PubMed
-
- Langer R. & Tirrell D. A. Designing materials for biology and medicine. Nature 428, 487–492 (2004). - PubMed
-
- Holzapfel B. M. et al. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 65, 581–603 (2013). - PubMed
-
- Busscher H. J. et al. Biomaterial-associated infection: locating the finish line in the race for the surface. Sci. Transl. Med. 4, 153rv10 (2012). - PubMed
-
- Bjarnsholt T., Ciofu O., Molin S., Givskov M. & Høiby N. Applying insights from biofilm biology to drug development - can a new approach be developed? Nat. Rev. Drug Discov. 12, 791–808 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

