Dorsoventral differences in Kv7/M-current and its impact on resonance, temporal summation and excitability in rat hippocampal pyramidal cells
- PMID: 25656084
- PMCID: PMC4386960
- DOI: 10.1113/jphysiol.2014.280826
Dorsoventral differences in Kv7/M-current and its impact on resonance, temporal summation and excitability in rat hippocampal pyramidal cells
Abstract
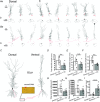
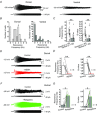
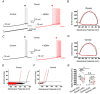
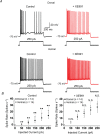
In rodent hippocampi, the connections, gene expression and functions differ along the dorsoventral (D-V) axis. CA1 pyramidal cells show increasing excitability along the D-V axis, although the underlying mechanism is not known. In the present study, we investigated how the M-current (IM ), caused by Kv7/M (KCNQ) potassium channels, and known to often control neuronal excitability, contributes to D-V differences in intrinsic properties of CA1 pyramidal cells. Using whole-cell patch clamp recordings and the selective Kv7/M blocker 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride (XE991) in hippocampal slices from 3- to 4-week-old rats, we found that: (i) IM had a stronger impact on subthreshold electrical properties in dorsal than ventral CA1 pyramidal cells, including input resistance, temporal summation of artificial synaptic potentials, and M-resonance; (ii) IM activated at more negative potentials (left-shifted) and had larger peak amplitude in the dorsal than ventral CA1; and (iii) the initial spike threshold (during ramp depolarizations) was elevated, and the medium after-hyperpolarization and spike frequency adaptation were increased (i.e. excitability was lower) in the dorsal rather than ventral CA1. These differences were abolished or reduced by application of XE991, indicating that they were caused by IM . Thus, it appears that IM has stronger effects in dorsal than in ventral rat CA1 pyramidal cells because of a larger maximal M-conductance and left-shifted activation curve in the dorsal cells. These mechanisms may contribute to D-V differences in the rate and phase coding of position by CA1 place cells, and may also enhance epileptiform activity in ventral CA1.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures






Comment in
-
Computational diversity in the hippocampus: a matter of components.J Physiol. 2015 Apr 1;593(7):1525-6. doi: 10.1113/jphysiol.2014.288456. J Physiol. 2015. PMID: 25828642 Free PMC article. No abstract available.
Similar articles
-
M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region.J Neurosci. 2007 Feb 21;27(8):1853-67. doi: 10.1523/JNEUROSCI.4463-06.2007. J Neurosci. 2007. PMID: 17314282 Free PMC article.
-
Kv7/M channel dysfunction produces hyperexcitability in hippocampal CA1 pyramidal cells of Fmr1 knockout mice.J Physiol. 2024 Aug;602(15):3769-3791. doi: 10.1113/JP285244. Epub 2024 Jul 8. J Physiol. 2024. PMID: 38976504
-
Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells.J Physiol. 2005 Aug 1;566(Pt 3):689-715. doi: 10.1113/jphysiol.2005.086835. Epub 2005 May 12. J Physiol. 2005. PMID: 15890705 Free PMC article.
-
M-channels modulate the intrinsic excitability and synaptic responses of layer 2/3 pyramidal neurons in auditory cortex.Biochem Biophys Res Commun. 2012 Oct 5;426(4):448-53. doi: 10.1016/j.bbrc.2012.08.057. Epub 2012 Aug 17. Biochem Biophys Res Commun. 2012. PMID: 22925893
-
Neural KCNQ (Kv7) channels.Br J Pharmacol. 2009 Apr;156(8):1185-95. doi: 10.1111/j.1476-5381.2009.00111.x. Epub 2009 Mar 9. Br J Pharmacol. 2009. PMID: 19298256 Free PMC article. Review.
Cited by
-
Examination of Diurnal Variation and Sex Differences in Hippocampal Neurophysiology and Spatial Memory.eNeuro. 2022 Nov 15;9(6):ENEURO.0124-22.2022. doi: 10.1523/ENEURO.0124-22.2022. Print 2022 Nov-Dec. eNeuro. 2022. PMID: 36265903 Free PMC article.
-
Increased Persistent Sodium Current Causes Neuronal Hyperexcitability in the Entorhinal Cortex of Fmr1 Knockout Mice.Cell Rep. 2016 Sep 20;16(12):3157-3166. doi: 10.1016/j.celrep.2016.08.046. Cell Rep. 2016. PMID: 27653682 Free PMC article.
-
Spatio-temporal heterogeneity in hippocampal metabolism in control and epilepsy conditions.Proc Natl Acad Sci U S A. 2021 Mar 16;118(11):e2013972118. doi: 10.1073/pnas.2013972118. Proc Natl Acad Sci U S A. 2021. PMID: 33692123 Free PMC article.
-
CA1 pyramidal cells have diverse biophysical properties, affected by development, experience, and aging.PeerJ. 2017 Sep 19;5:e3836. doi: 10.7717/peerj.3836. eCollection 2017. PeerJ. 2017. PMID: 28948109 Free PMC article.
-
Distinct Properties of Long-Term Potentiation in the Dentate Gyrus along the Dorsoventral Axis: Influence of Age and Inhibition.Sci Rep. 2017 Jul 11;7(1):5157. doi: 10.1038/s41598-017-05358-1. Sci Rep. 2017. PMID: 28698637 Free PMC article.
References
-
- Alle H, Ostroumov K, Geiger J. Storm JF. M-current, persistent Na+ current, and subthreshold resonance recorded in mossy fiber boutons. 2009. (MFBs) in rat hippocampus. Program No. 42.2/D10. 2009 Neuroscience Meeting Planner. Society for Neuroscience, Chicago, IL, USA. Online. Ref Type: Abstract.
-
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, Zhang WN, Pothuizen HHJ. Feldon J. Regional dissociations within the hippocampus – memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

