UPR, autophagy, and mitochondria crosstalk underlies the ER stress response
- PMID: 25656104
- PMCID: PMC4340752
- DOI: 10.1016/j.tibs.2015.01.002
UPR, autophagy, and mitochondria crosstalk underlies the ER stress response
Abstract
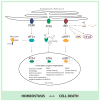
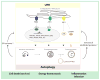
Cellular stress, induced by external or internal cues, activates several well-orchestrated processes aimed at either restoring cellular homeostasis or committing to cell death. Those processes include the unfolded protein response (UPR), autophagy, hypoxia, and mitochondrial function, which are part of the global endoplasmic reticulum (ER) stress (ERS) response. When one of the ERS elements is impaired, as often occurs under pathological conditions, overall cellular homeostasis may be perturbed. Further, activation of the UPR could trigger changes in mitochondrial function or autophagy, which could modulate the UPR, exemplifying crosstalk processes. Among the numerous factors that control the magnitude or duration of these processes are ubiquitin ligases, which govern overall cellular stress outcomes. Here we summarize crosstalk among the fundamental processes governing ERS responses.
Keywords: ER stress; UPR; autophagy; hypoxia; mitochondria; ubiquitin.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
References
-
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. - PubMed
-
- Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006;18:444–452. - PubMed
-
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

