Labour induction with prostaglandins: a systematic review and network meta-analysis
- PMID: 25656228
- PMCID: PMC4353287
- DOI: 10.1136/bmj.h217
Labour induction with prostaglandins: a systematic review and network meta-analysis
Abstract
Objectives: To assess the effectiveness and safety of prostaglandins used for labour induction.
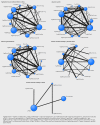
Design: Systematic review with Bayesian network meta-analysis
Data sources: The Cochrane Pregnancy and Childbirth Group's Database of Trials (which incorporates the results of a broad generic search for all pregnancy and postpartum trials). Sources included are CENTRAL, Medline, Embase, NHS Economic Evaluation Database, CINAHL, relevant journals, conference proceedings, and registries of ongoing trials.
Eligibility criteria for selecting studies: Randomised clinical trials of prostaglandin or prostaglandin analogues used for third trimester cervical ripening or labour induction versus placebo or no treatment, alternative prostaglandin dose or administration, or a different type of prostaglandin. We included studies recruiting women with a viable fetus, but had no other restrictions relating to indication for labour induction or language of publication. Outcomes assessed were serious neonatal morbidity (trialist defined) or perinatal death; serious maternal morbidity (trialist defined) or death; vaginal delivery not achieved within 24 hours, caesarean section, and uterine hyperstimulation with fetal heart rate changes.
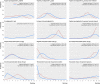
Results: 280 randomised clinical trials were included (48 068 women) in the review. Maternal and neonatal mortality and serious morbidity were rarely reported and are summarized narratively. Unresolved inconsistency was observed for the hyperstimulation outcome. Relative to placebo, the odds of failing to achieve a vaginal delivery were lowest for vaginal misoprostol (≥50 µg) (odds ratio 0.06 (95% credible interval 0.02 to 0.12)), with a 39% absolute probability of event (95% credible interval 1% to 94%). Compared with placebo, odds of caesarean section were lowest for titrated oral misoprostol solution (<50 µg) (odds ratio 0.65 (0.49 to 0.83)), with an absolute probability of event of 15% (3% to 40%).
Conclusions: Low dose(<50 µg) titrated oral misoprostol solution had the lowest probability of caesarean section, whereas vaginal misprostol (≥50 µg) had the highest probability of achieving a vaginal delivery within 24 hours. These findings have important implications for a series of current national and international guidelines for induction of labour and future research in this area.
Systematic review registration: PROSPERO 2013:CRD42013005116.
© Alfirevic et al 2015.
Figures


References
-
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. No. 107. Induction of Labor. Obstet Gynecol 2009;114:386-97.
-
- Chauhan SP, Ananth CV. Induction of labor in the United States: a critical appraisal of appropriateness and reducibility. Semin Perinatol 2012;36:336-43. - PubMed
-
- National Institute for Health and Care Excellence. Induction of labour. (Clinical guideline 70). NICE, 2008. www.nice.org.uk/guidance/cg70.
-
- Tang J, Kapp N, Dragoman M, de Souza JP. WHO recommendations for misoprostol use for obstetric and gynecologic indications. Int J Gynaecol Obstet 2013;121:186-9. - PubMed
-
- Austin SC, Sanchez-Ramos L, Adair CD. Labor induction with intravaginal misoprostol compared with the dinoprostone vaginal insert: a systematic review and metaanalysis. Am J Obstet Gynecol 2010;202:624.e1-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
