Fgfr2 is integral for bladder mesenchyme patterning and function
- PMID: 25656370
- PMCID: PMC4398839
- DOI: 10.1152/ajprenal.00624.2014
Fgfr2 is integral for bladder mesenchyme patterning and function
Retraction in
-
Retraction.Am J Physiol Renal Physiol. 2016 Jul 1;311(1):F239. doi: 10.1152/ajprenal.zh2-7964-retr.2016. Am J Physiol Renal Physiol. 2016. PMID: 27390325 Free PMC article. No abstract available.
Abstract
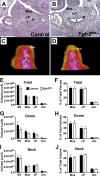
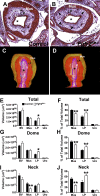
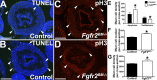
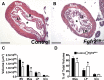
While urothelial signals, including sonic hedgehog (Shh), drive bladder mesenchyme differentiation, it is unclear which pathways within the mesenchyme are critical for its development. Studies have shown that fibroblast growth factor receptor (Fgfr)2 is necessary for kidney and ureter mesenchymal development. The objective of the present study was to determine the role of Fgfr2 in the bladder mesenchyme. We used Tbx18cre mice to delete Fgfr2 in the bladder mesenchyme (Fgfr2(BM-/-)). We performed three-dimensional reconstructions, quantitative real-time PCR, in situ hybridization, immunolabeling, ELISAs, immunoblot analysis, void stain on paper, ex vivo bladder sheet assays, and in vivo decerebrated cystometry. Compared with control bladders, embryonic day 16.5 (E16.5) Fgfr2(BM-/-) bladders had thin muscle layers with less α-smooth muscle actin and thickened lamina propria with increased collagen type Ia and IIIa that intruded into the muscle. The reciprocal changes in mutant layer thicknesses appeared partly due to a cell fate switch. From postnatal days 1 to 30, Fgfr2(BM-/-) bladders demonstrated progressive muscle loss and increased collagen expression. Postnatal Fgfr2(BM-/-) bladder sheets exhibited decreased agonist-mediated contractility and increased passive stretch tension versus control bladder sheets. Cystometry revealed high baseline and threshold pressures and shortened intercontractile intervals in Fgfr2(BM-/-) versus control bladders. Mechanistically, whereas Shh expression appeared normal, mRNA and protein readouts of hedgehog activity were increased in E16.5 Fgfr2(BM-/-) versus control bladders. Moreover, E16.5 Fgfr2(BM-/-) bladders exhibited higher levels of Cdo and Boc, hedgehog coreceptors that enhance sensitivity to Shh, compared with control bladders. In conclusion, loss of Fgfr2 in the bladder mesenchyme leads to abnormal bladder morphology and decreased compliance and contractility.
Keywords: bladder development; bladder dysfunction; fibroblast growth factor receptor 2.
Copyright © 2015 the American Physiological Society.
Figures



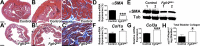

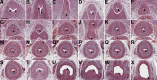


Comment in
-
Findings of Research Misconduct.NIH Guide Grants Contracts (Bethesda). 2016 May 20:NOT-OD-16-090. NIH Guide Grants Contracts (Bethesda). 2016. PMID: 27220120 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2016 May 11;81(91):29278. Fed Regist. 2016. PMID: 27737276 Free PMC article. No abstract available.
References
-
- Avlan D, Gundogdu G, Taskinlar H, Delibas A, Nayci A. Relationships among vesicoureteric reflux, urinary tract infection and renal injury in children with non-neurogenic lower urinary tract dysfunction. J Pediatr Urol 7: 612–615, 2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

