Adrenocorticotropic Hormone and PI3K/Akt Inhibition Reduce eNOS Phosphorylation and Increase Cortisol Biosynthesis in Long-Term Hypoxic Ovine Fetal Adrenal Cortical Cells
- PMID: 25656500
- PMCID: PMC5933094
- DOI: 10.1177/1933719115570899
Adrenocorticotropic Hormone and PI3K/Akt Inhibition Reduce eNOS Phosphorylation and Increase Cortisol Biosynthesis in Long-Term Hypoxic Ovine Fetal Adrenal Cortical Cells
Abstract
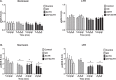
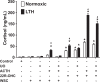
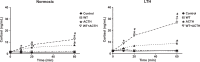
This study was designed to determine the role of the MEK/ERK1/2 and PI3K/Akt pathways in cortisol production and endothelial nitric oxide synthase (eNOS) phosphorylation (peNOS) in the ovine fetal adrenal in response to long-term hypoxia (LTH). Pregnant ewes were maintained at high altitude (3820 m) for the last 100 days of gestation (dGa). At 138 to 142 dGa, fetal adrenal cortical cells (FACs) were collected from LTH and age-matched normoxic fetuses. Cortisol production and peNOS were measured in response to pretreatment with the MEK/ERK1/2 pathway inhibitor UO126 (UO) and adrenocorticotropic hormone (ACTH) stimulation. UO126 reduced ACTH-stimulated cortisol in both normoxic and LTH FACs. UO126 alone or in combination with ACTH reduced peNOS in the normoxic group, while ACTH alone or ACTH + UO inhibited peNOS in LTH FACs. Additionally, cortisol was measured in response to pretreatment with UO and treatment with 22R-hydroxycholesterol (22R-OHC) or water-soluble cholesterol (WSC) with and without ACTH stimulation. UO126 had no effect on 22R-OHC-treated cells, but reduced cortisol in cells treated with WSC and/or ACTH. Cortisol and peNOS were also measured in response to pretreatment with PI3K/Akt pathway inhibitor Wortmannin (WT) and ACTH stimulation. Wortmannin further increased cortisol under ACTH-stimulated conditions and, like ACTH, reduced peNOS in LTH but not normoxic FACs. Together, these data suggest that in LTH FACs MEK/ERK1/2 does not regulate peNOS but that UO acts downstream from eNOS, possibly at cholesterol transport, to affect cortisol production in LTH FACs, while the PI3K/Akt pathway, along with ACTH, regulates peNOS and plays a role in the fetal adaptation to LTH in FACs.
Keywords: adrenocorticotropic hormone; hypoxia; sheep.
© The Author(s) 2015.
Conflict of interest statement
Figures
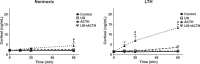
Similar articles
-
Extracellular signal-regulated kinases (ERK1/2) signaling pathway plays a role in cortisol secretion in the long-term hypoxic ovine fetal adrenal near term.Am J Physiol Regul Integr Comp Physiol. 2013 Apr 15;304(8):R636-43. doi: 10.1152/ajpregu.00318.2012. Epub 2013 Feb 20. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23427082 Free PMC article.
-
Expression of StAR and Key Genes Regulating Cortisol Biosynthesis in Near Term Ovine Fetal Adrenocortical Cells: Effects of Long-Term Hypoxia.Reprod Sci. 2018 Feb;25(2):230-238. doi: 10.1177/1933719117707056. Epub 2017 May 3. Reprod Sci. 2018. PMID: 28468567 Free PMC article.
-
Nitric oxide inhibits ACTH-induced cortisol production in near-term, long-term hypoxic ovine fetal adrenocortical cells.Reprod Sci. 2010 Oct;17(10):955-62. doi: 10.1177/1933719110376092. Epub 2010 Aug 16. Reprod Sci. 2010. PMID: 20713972 Free PMC article.
-
Adrenocortical and adipose responses to high-altitude-induced, long-term hypoxia in the ovine fetus.J Pregnancy. 2012;2012:681306. doi: 10.1155/2012/681306. Epub 2012 May 14. J Pregnancy. 2012. PMID: 22666594 Free PMC article. Review.
-
Altitude, attitude and adaptation.Adv Exp Med Biol. 2014;814:147-57. doi: 10.1007/978-1-4939-1031-1_13. Adv Exp Med Biol. 2014. PMID: 25015808 Free PMC article. Review.
Cited by
-
Fetal endocrine and metabolic adaptations to hypoxia: the role of the hypothalamic-pituitary-adrenal axis.Am J Physiol Endocrinol Metab. 2015 Sep 1;309(5):E429-39. doi: 10.1152/ajpendo.00126.2015. Epub 2015 Jul 14. Am J Physiol Endocrinol Metab. 2015. PMID: 26173460 Free PMC article. Review.
-
Influence of nitric oxide signaling mechanisms in cancer.Int J Immunopathol Pharmacol. 2022 Jan-Dec;36:3946320221135454. doi: 10.1177/03946320221135454. Int J Immunopathol Pharmacol. 2022. PMID: 36260949 Free PMC article.
References
-
- Imamura T, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters endocrine and physiologic responses to umbilical cord occlusion in the ovine fetus. J Soc Gynecol Investig. 2004;11 (3):131–140. - PubMed
-
- Adachi K, Umezaki H, Kaushal KM, Ducsay CA. Long-term hypoxia alters ovine fetal endocrine and physiological responses to hypotension. Am J Physiol Regul Integr Comp Physiol. 2004;287 (1):R209–R217. - PubMed
-
- Bocking AD, McMillen IC, Harding R, Thorburn GD. Effect of reduced uterine blood flow on fetal and maternal cortisol. J Dev Physiol.1986;8 (4):237–245. - PubMed
-
- Boddy K, Jones CT, Mantell C, Ratcliffe JG, Robinson JS. Changes in plasma ACTH and corticosteroid of the maternal and fetal sheep during hypoxia. Endocrinology. 1974;94 (2):588–591. - PubMed
-
- Jones CT, Boddy K, Robinson JS, Ratcliffe JG. Developmental changes in the responses of the adrenal glands of foetal sheep to endogenous adrenocorticotrophin, as indicated by hormone responses to hypoxaemia. J Endocrinol. 1977;72 (3):279–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

