Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance
- PMID: 25656600
- PMCID: PMC4876855
- DOI: 10.1016/S1473-3099(14)71081-3
Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance
Abstract
Background: In 2000, seven-valent pneumococcal conjugate vaccine (PCV7) was introduced in the USA and resulted in dramatic reductions in invasive pneumococcal disease (IPD) and moderate increases in non-PCV7 type IPD. In 2010, PCV13 replaced PCV7 in the US immunisation schedule. We aimed to assess the effect of use of PCV13 in children on IPD in children and adults in the USA.
Methods: We used laboratory-based and population-based data on incidence of IPD from the Active Bacterial Core surveillance (part of the Centers for Disease Control and Prevention's Emerging Infections Program) in a time-series model to compare rates of IPD before and after the introduction of PCV13. Cases of IPD between July 1, 2004, and June 30, 2013, were classified as being caused by the PCV13 serotypes against which PCV7 has no effect (PCV13 minus PCV7). In a time-series model, we used an expected outcomes approach to compare the reported incidence of IPD to that which would have been expected if PCV13 had not replaced PCV7.
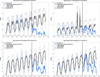
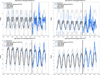
Findings: Compared with incidence expected among children younger than 5 years if PCV7 alone had been continued, incidence of IPD overall declined by 64% (95% interval estimate [95% IE] 59-68) and IPD caused by PCV13 minus PCV7 serotypes declined by 93% (91-94), by July, 2012, to June, 2013. Among adults, incidence of IPD overall also declined by 12-32% and IPD caused by PCV13 minus PCV7 type IPD declined by 58-72%, depending on age. We estimated that over 30 000 cases of IPD and 3000 deaths were averted in the first 3 years after the introduction of PCV13.
Interpretation: PCV13 reduced IPD across all age groups when used routinely in children in the USA. These findings provide reassurance that, similar to PCV7, PCVs with additional serotypes can also prevent transmission to unvaccinated populations.
Funding: Centers for Disease Control and Prevention.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
For all other authors, we declare that we have no conflicts of interest.
Figures



Comment in
-
PCV13 in the USA: early successes and potential challenges.Lancet Infect Dis. 2015 Mar;15(3):254-6. doi: 10.1016/S1473-3099(15)70018-6. Epub 2015 Feb 3. Lancet Infect Dis. 2015. PMID: 25656599 No abstract available.
References
-
- Centers for Disease Control and Prevention. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2000;49(RR-9):1–35. - PubMed
-
- Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. - PubMed
-
- Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118(3):865–873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

