Rosiglitazone via PPARγ-dependent suppression of oxidative stress attenuates endothelial dysfunction in rats fed homocysteine thiolactone
- PMID: 25656735
- PMCID: PMC4395197
- DOI: 10.1111/jcmm.12510
Rosiglitazone via PPARγ-dependent suppression of oxidative stress attenuates endothelial dysfunction in rats fed homocysteine thiolactone
Abstract
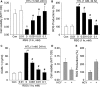
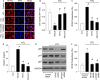
To explore whether rosiglitazone (RSG), a selective peroxisome proliferator-activated receptor γ (PPARγ) agonist, exerts beneficial effects on endothelial dysfunction induced by homocysteine thiolactone (HTL) and to investigate the potential mechanisms. Incubation of cultured human umbilical vein endothelial cells with HTL (1 mM) for 24 hrs significantly reduced cell viabilities assayed by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, as well as enhanced productions of reactive oxygen species, activation of nuclear factor kappa B, and increased intercellular cell adhesion molecule-1 secretion. Pre-treatment of cells with RSG (0.001-0.1 mM), pyrollidine dithiocarbamate (PDTC, 0.1 mM) or apocynin (0.1 mM) for 1 hr reversed these effects induced by HTL. Furthermore, co-incubation with GW9662 (0.01 mM) abolished the protective effects of RSG on HTL-treated cells. In ex vivo experiments, exposure of isolated aortic rings from. rats to HTL (1 mM) for 1 hr dramatically impaired acetylcholine-induced endothelium-dependent relaxation, reduced release of nitric oxide and activity of superoxide dismutase, and increased malondialdehyde content in aortic tissues. Preincubation of aortic rings with RSG (0.1, 0.3, 1 mM), PDTC or apocynin normalized the disorders induced by HTL. In vivo analysis indicated that administration of RSG (20 mg/kg/d) remarkably suppressed oxidative stress and prevented endothelial dysfunction in rats fed HTL (50 mg/kg/d) for 8 weeks. RSG improves endothelial functions in rats fed HTL, which is related to PPARγ-dependent suppression of oxidative stress.
Keywords: endothelial dysfunction; homocysteine thiolactone; oxidative stress; rosiglitazone; vascular ageing.
© 2015 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures
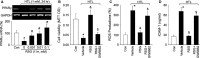

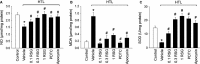

References
-
- Kolodziejczyk-Czepas J, Talar B, Nowak P, et al. Homocysteine and its thiolactone impair plasmin activity induced by urokinase or streptokinase in vitro. Int J Biol Macromol. 2012;50:754–8. - PubMed
-
- Liu Y, Tian T, Zhang H, et al. The effect of homocysteine-lowering therapy with folic acid on flow-mediated vasodilation in patients with coronary artery disease: a meta-analysis of randomized controlled trials. Atherosclerosis. 2014;235:31–5. - PubMed
-
- de Andrade CR, Fukada SY, Olivon VC, et al. Alpha1d-adrenoceptor-induced relaxation on rat carotid artery is impaired during the endothelial dysfunction evoked in the early stages of hyperhomocysteinemia. Eur J Pharmacol. 2006;543:83–91. - PubMed
-
- Kersten S, Desvergne B, Wahli W. Roles of ppars in health and disease. Nature. 2000;405:421–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

