Sweets for a bitter end: lung cancer cell-surface protein glycosylation mediates metastatic colonization
- PMID: 25656895
- PMCID: PMC4340588
- DOI: 10.1158/2159-8290.CD-15-0013
Sweets for a bitter end: lung cancer cell-surface protein glycosylation mediates metastatic colonization
Abstract
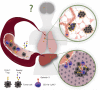
Glycosylation is one of the most predominant forms of cell-surface protein modifications, yet its deregulation in cancer and contribution to tumor microenvironment interactions remain poorly understood. In this issue of Cancer Discovery, Reticker-Flynn and Bhatia characterize an enzymatic switch in lung cancer cells that triggers aberrant surface protein glycosylation patterns, adhesion to lectins on the surface of inflammatory cells, and subsequent metastatic colonization of the liver.
©2015 American Association for Cancer Research.
Figures
Comment on
-
Aberrant glycosylation promotes lung cancer metastasis through adhesion to galectins in the metastatic niche.Cancer Discov. 2015 Feb;5(2):168-81. doi: 10.1158/2159-8290.CD-13-0760. Epub 2014 Nov 24. Cancer Discov. 2015. PMID: 25421439 Free PMC article.
References
-
- Bull C, Stoel MA, den Brok MH, Adema GJ. Sialic acids sweeten a tumor's life. Cancer Res. 2014;74:3199–204. - PubMed
-
- Liang Y, Ma T, Thakur A, Yu H, Gao L, Shi P, et al. Differentially expressed glycosylated patterns of alpha-1-antitrypsin as serum biomarkers for the diagnosis of lung cancer. Glycobiology. 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

