Developing de novo human artificial chromosomes in embryonic stem cells using HSV-1 amplicon technology
- PMID: 25657030
- PMCID: PMC4365269
- DOI: 10.1007/s10577-014-9456-2
Developing de novo human artificial chromosomes in embryonic stem cells using HSV-1 amplicon technology
Abstract
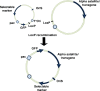
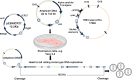
De novo artificial chromosomes expressing genes have been generated in human embryonic stem cells (hESc) and are maintained following differentiation into other cell types. Human artificial chromosomes (HAC) are small, functional, extrachromosomal elements, which behave as normal chromosomes in human cells. De novo HAC are generated following delivery of alpha satellite DNA into target cells. HAC are characterized by high levels of mitotic stability and are used as models to study centromere formation and chromosome organisation. They are successful and effective as gene expression vectors since they remain autonomous and can accommodate larger genes and regulatory regions for long-term expression studies in cells unlike other viral gene delivery vectors currently used. Transferring the essential DNA sequences for HAC formation intact across the cell membrane has been challenging for a number of years. A highly efficient delivery system based on HSV-1 amplicons has been used to target DNA directly to the ES cell nucleus and HAC stably generated in human embryonic stem cells (hESc) at high frequency. HAC were detected using an improved protocol for hESc chromosome harvesting, which consistently produced high-quality metaphase spreads that could routinely detect HAC in hESc. In tumour cells, the input DNA often integrated in the host chromosomes, but in the host ES genome, it remained intact. The hESc containing the HAC formed embryoid bodies, generated teratoma in mice, and differentiated into neuronal cells where the HAC were maintained. The HAC structure and chromatin composition was similar to the endogenous hESc chromosomes. This review will discuss the technological advances in HAC vector delivery using HSV-1 amplicons and the improvements in the identification of de novo HAC in hESc.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

