Targeting Filarial Abl-like Kinases: Orally Available, Food and Drug Administration-Approved Tyrosine Kinase Inhibitors Are Microfilaricidal and Macrofilaricidal
- PMID: 25657255
- PMCID: PMC4539898
- DOI: 10.1093/infdis/jiv065
Targeting Filarial Abl-like Kinases: Orally Available, Food and Drug Administration-Approved Tyrosine Kinase Inhibitors Are Microfilaricidal and Macrofilaricidal
Abstract
Background: Elimination of onchocerciasis and lymphatic filariasis is targeted for 2020. Given the coincident Loa loa infections in Central Africa and the potential for drug resistance development, the need for new microfilaricides and macrofilaricides has never been greater. With the genomes of L. loa, Onchocerca volvulus, Wuchereria bancrofti, and Brugia malayi available, new drug targets have been identified.
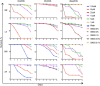
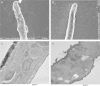
Methods: The effects of the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib on B. malayi adult males, adult females, L3 larvae, and microfilariae were assessed using a wide dose range (0-100 µM) in vitro.
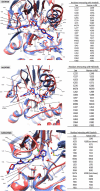
Results: For microfilariae, median inhibitory concentrations (IC50 values) on day 6 were 6.06 µM for imatinib, 3.72 µM for dasatinib, and 81.35 µM for nilotinib; for L3 larvae, 11.27 µM, 13.64 µM, and 70.98 µM, respectively; for adult males, 41.6 µM, 3.87 µM, and 68.22 µM, respectively; and for adult females, 42.89 µM, 9.8 µM, and >100 µM, respectively. Three-dimensional modeling suggests how these tyrosine kinase inhibitors bind and inhibit filarial protein activity.
Conclusions: Given the safety of imatinib in humans, plans are underway for pilot clinical trials to assess its efficacy in patients with filarial infections.
Keywords: Brugia malayi; Loa loa; Wuchereria bancrofti; filaria; lymphatic filariasis; macrofilaricide; mass drug administration; microfilaricide; onchocerciasis.
Published by Oxford University Press on behalf of the Infectious Diseases Society of America 2015. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures


Comment in
-
Adding "Filaricide" to the Gleevec Portfolio.J Infect Dis. 2015 Sep 1;212(5):677-80. doi: 10.1093/infdis/jiv062. Epub 2015 Feb 5. J Infect Dis. 2015. PMID: 25657258 No abstract available.
References
-
- Fenwick A. The global burden of neglected tropical diseases. Public Health 2012; 126:233–6. - PubMed
-
- World Health Organization Global programme to eliminate lymphatic filariasis: progress report on mass drug administration, 2010. Wkly Epidemiol Rec 2011; 86:377–88. - PubMed
-
- Bockarie MJ, Taylor MJ, Gyapong JO. Current practices in the management of lymphatic filariasis. Expert Rev Anti Infect Ther 2009; 7:595–605. - PubMed
-
- Gyapong JO, Kumaraswami V, Ottesesen E. Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opin Pharmacother 2005; 6:179–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

