Nanoparticle-mediated expression of a Wnt pathway inhibitor ameliorates ocular neovascularization
- PMID: 25657312
- PMCID: PMC4376607
- DOI: 10.1161/ATVBAHA.114.304627
Nanoparticle-mediated expression of a Wnt pathway inhibitor ameliorates ocular neovascularization
Abstract
Objective: The deficiency of very low-density lipoprotein receptor resulted in Wnt signaling activation and neovascularization in the retina. The present study sought to determine whether the very low-density lipoprotein receptor extracellular domain (VLN) is responsible for the inhibition of Wnt signaling in ocular tissues.
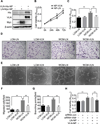
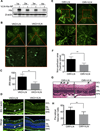
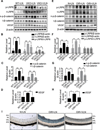
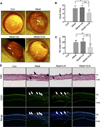
Approach and results: A plasmid expressing the soluble VLN was encapsulated with poly(lactide-co-glycolide acid) to form VLN nanoparticles (VLN-NP). Nanoparticles containing a plasmid expressing the low-density lipoprotein receptor extracellular domain nanoparticle were used as negative control. MTT, modified Boyden chamber, and Matrigel (™) assays were used to evaluate the inhibitory effect of VLN-NP on Wnt3a-stimulated endothelial cell proliferation, migration, and tube formation. Vldlr(-/-) mice, oxygen-induced retinopathy, and alkali burn-induced corneal neovascularization models were used to evaluate the effect of VLN-NP on ocular neovascularization. Wnt reporter mice (BAT-gal), Western blotting, and luciferase assay were used to evaluate Wnt pathway activity. Our results showed that VLN-NP specifically inhibited Wnt3a-induced endothelial cell proliferation, migration, and tube formation. Intravitreal injection of VLN-NP inhibited abnormal neovascularization in Vldlr(-/-), oxygen-induced retinopathy, and alkali burn-induced corneal neovascularization models, compared with low-density lipoprotein receptor extracellular domain nanoparticle. VLN-NP significantly inhibited the phosphorylation of low-density lipoprotein receptor-related protein 6, the accumulation of β-catenin, and the expression of vascular endothelial growth factor in vivo and in vitro.
Conclusions: Taken together, these results suggest that the soluble VLN is a negative regulator of the Wnt pathway and has antiangiogenic activities. Nanoparticle-mediated expression of VLN may thus represent a novel therapeutic approach to treat pathological ocular angiogenesis and potentially other vascular diseases affected by Wnt signaling.
Keywords: VLDLR; Wnt; eye; nanoparticle; neovascularization.
© 2015 American Heart Association, Inc.
Figures

Comment in
-
Wnting out ocular neovascularization: using nanoparticle delivery of very-low density lipoprotein receptor extracellular domain as Wnt pathway inhibitor in the retina.Arterioscler Thromb Vasc Biol. 2015 May;35(5):1046-7. doi: 10.1161/ATVBAHA.115.305395. Arterioscler Thromb Vasc Biol. 2015. PMID: 25903649 Free PMC article. No abstract available.
Similar articles
-
Wnting out ocular neovascularization: using nanoparticle delivery of very-low density lipoprotein receptor extracellular domain as Wnt pathway inhibitor in the retina.Arterioscler Thromb Vasc Biol. 2015 May;35(5):1046-7. doi: 10.1161/ATVBAHA.115.305395. Arterioscler Thromb Vasc Biol. 2015. PMID: 25903649 Free PMC article. No abstract available.
-
Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization.J Biol Chem. 2007 Nov 23;282(47):34420-8. doi: 10.1074/jbc.M611289200. Epub 2007 Sep 23. J Biol Chem. 2007. PMID: 17890782
-
Inhibition of ocular neovascularization by a novel peptide derived from human placenta growth factor-1.Acta Ophthalmol. 2012 Nov;90(7):e512-23. doi: 10.1111/j.1755-3768.2012.02476.x. Epub 2012 Sep 20. Acta Ophthalmol. 2012. PMID: 22994140
-
[Cell biology of intraocular vascular diseases].Nippon Ganka Gakkai Zasshi. 1999 Dec;103(12):923-47. Nippon Ganka Gakkai Zasshi. 1999. PMID: 10643294 Review. Japanese.
-
Mediators of ocular angiogenesis.J Genet. 2009 Dec;88(4):495-515. doi: 10.1007/s12041-009-0068-0. J Genet. 2009. PMID: 20090210 Free PMC article. Review.
Cited by
-
Anti-angiogenic effect of a humanized antibody blocking the Wnt/β-catenin signaling pathway.Microvasc Res. 2018 Sep;119:29-37. doi: 10.1016/j.mvr.2018.03.011. Epub 2018 Apr 7. Microvasc Res. 2018. PMID: 29630973 Free PMC article.
-
Current and emerging therapies for corneal neovascularization.Ocul Surf. 2018 Oct;16(4):398-414. doi: 10.1016/j.jtos.2018.06.004. Epub 2018 Jun 20. Ocul Surf. 2018. PMID: 29908870 Free PMC article. Review.
-
Curcumin and Wnt/β‑catenin signaling in exudative age‑related macular degeneration (Review).Int J Mol Med. 2022 Jun;49(6):79. doi: 10.3892/ijmm.2022.5135. Epub 2022 Apr 21. Int J Mol Med. 2022. PMID: 35445729 Free PMC article. Review.
-
Oxidative stress upregulates Wnt signaling in human retinal microvascular endothelial cells through activation of disheveled.J Cell Biochem. 2019 Aug;120(8):14044-14054. doi: 10.1002/jcb.28679. Epub 2019 Apr 8. J Cell Biochem. 2019. PMID: 30963607 Free PMC article.
-
The functional role of decorin in corneal neovascularization in vivo.Exp Eye Res. 2021 Jun;207:108610. doi: 10.1016/j.exer.2021.108610. Epub 2021 Apr 30. Exp Eye Res. 2021. PMID: 33940009 Free PMC article.
References
-
- Rathmann W, Giani G. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27:2568–2569. author reply 2569. - PubMed
-
- Bunker DJ, George RJ, Kumar RJ, Maitz P. Alkali-related ocular burns: A case series and review. Journal of burn care & research : official publication of the American Burn Association. 2013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

