Severe cutaneous adverse drug reactions: a clinicoepidemiological study
- PMID: 25657416
- PMCID: PMC4318022
- DOI: 10.4103/0019-5154.147834
Severe cutaneous adverse drug reactions: a clinicoepidemiological study
Abstract
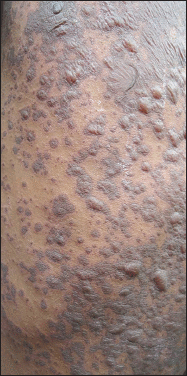
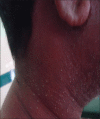
Background: Drug eruptions range from transient erythema to the life threatening severe cutaneous adverse reactions (SCAR) that encompass Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms complex (DRESS).
Aims and objectives: To study the clinical and epidemiological aspects of cutaneous adverse drug reactions (CADR).
Materials and methods: Ethical clearance was obtained from the institutional ethics committee. All patients admitted in the Dermatology ward of our tertiary care hospital with CADR (those who fit in the category of probable or possible drug reaction as per WHO casuality assessment) from first September 2011 to 31(st) August 2012 were included in this cross sectional study after obtaining written informed consent. The drug reaction patterns observed in the study population were determined and the common offending drugs were identified.
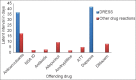
Results: In the study, population of males outnumbered females and the majority were between 46 and 60 years of age. The commonest reaction pattern observed was SJS- TEN spectrum of illness and aromatic anticonvulsants were the common offending drugs. Prompt withdrawal of the culprit drug and administration of systemic steroids with or without I/V Ig reverted the adverse reaction in all except one.
Conclusion: Severe drug reactions predominated as the study population was comprised of inpatients of a tertiary referral centre. Though; previous authors had reported a mortality rate of up to 20% in DRESS, all our patients with this reaction pattern, responded well to treatment. The mortality rate among TEN cases was much lower than the previous reports. Early diagnosis, prompt withdrawal of the suspected drug, careful monitoring for development of complications and immediate intervention can improve the prognosis of CADR.
Keywords: Drug reaction; drug reaction with eosinophilia and systemic symptoms complex; epidemiology.
Conflict of interest statement
Figures


References
-
- Roujeau JC, Allanore L, Liss Y, Mockenhaupt M. Severe Cutaneous Adverse Reactions to Drugs (SCAR): Definitions, diagnostic criteria, genetic predisposition. Dermatol Sinica. 2009;27:203–9.
-
- Bilimoria FE, Shah BJ. 3rd ed. Vol. 2. Mumbai: Bhalani Publishing House; 2008. Drug Reactions. IADVL Text Book of Dermatology; pp. 1633–86.
-
- Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis, and management. Lancet. 2000;356:1255–9. - PubMed
-
- Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice study II. N Eng J Med. 1991;324:377–84. - PubMed
-
- Hafner JW, Belknap SM, Squillante MD, Bucheit KA. Adverse drug events in emergency department patients. Ann Emerg Med. 2002;39:258–67. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
