The spino-bulbar-cerebellar pathway: organization and neurochemical properties of spinal cells that project to the lateral reticular nucleus in the rat
- PMID: 25657619
- PMCID: PMC4303139
- DOI: 10.3389/fnana.2015.00001
The spino-bulbar-cerebellar pathway: organization and neurochemical properties of spinal cells that project to the lateral reticular nucleus in the rat
Abstract
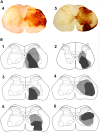
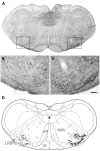
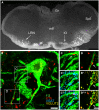
In addition to classical spinocerebellar pathways, the cerebellum receives information from the spinal cord indirectly via spino-bulbar-cerebellar systems. One of the structures in this pathway is the lateral reticular nucleus (LRt). We performed series of experiments to investigate the organization and neurotransmitter content of spinoreticular tract (SRT) neurons in the lumbar spinal cord that project to the LRt. Three rats received injections of the b subunit of Cholera toxin (CTb) or Fluorogold (FG) within the left and right LRt. The majority of SRT cells (56-61%) were found within the contralateral medial intermediate gray matter where small numbers (7-10%) of double-labeled cells were also present on both sides of the cord. Six rats received unilateral spinal injections of CTb to label spinal projections to the LRt. Injections of FG were made also into the anterior lobe of the cerebellum to label LRt pre-cerebellar neurons. Terminals were found mainly ipsilateral to spinal injection sites within the central and ventrolateral regions of the LRt. Immunocytochemical analysis of SRT terminals revealed that the majority (75%) were contained vesicular glutamate transporter 2 but a minority (20%) contained the vesicular GABA transporter. The inhibitory subpopulation was found to be GABAergic, glycinergic, or contained both transmitters. Inhibitory and excitatory terminals were present within overlapping regions of the nucleus. Most CTb terminals contacting LRt pre-cerebellar neurons were excitatory (80%) whereas a minority were inhibitory and most cells (88%) received contacts from both inhibitory and excitatory terminals. This study shows that SRT axons in the LRt have the capacity to exert direct excitatory and inhibitory actions on LRt pre-cerebellar neurons. Thus spinal cord input has the capacity to facilitate or depress the activity of individual LRt cells which in turn adjust activity in the cerebellum to produce coordinated motor behaviors.
Keywords: cerebellum; motor control; neuroanatomy; neurotransmitters; spinal cord.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources

