Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages
- PMID: 25657646
- PMCID: PMC4303141
- DOI: 10.3389/fimmu.2014.00683
Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages
Abstract
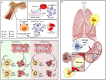
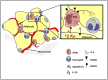
The explosion of new information in recent years on the origin of macrophages in the steady-state and in the context of inflammation has opened up numerous new avenues of investigation and possibilities for therapeutic intervention. In contrast to the classical model of macrophage development, it is clear that tissue-resident macrophages can develop from yolk sac-derived erythro-myeloid progenitors, fetal liver progenitors, and bone marrow-derived monocytes. Under both homeostatic conditions and in response to pathophysiological insult, the contribution of these distinct sources of macrophages varies significantly between tissues. Furthermore, while all of these populations of macrophages appear to be capable of adopting the polarized M1/M2 phenotypes, their respective contribution to inflammation, resolution of inflammation, and tissue repair remains poorly understood and is likely to be tissue- and disease-dependent. A better understanding of the ontology and polarization capacity of macrophages in homeostasis and disease will be essential for the development of novel therapies that target the inherent plasticity of macrophages in the treatment of acute and chronic inflammatory disease.
Keywords: Kupffer cells; M1M2; adipose tissue macrophages; hepatic steatosis; microglia; neurodegenerative disease; obesity; tissue-resident macrophages.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

