Sensing of Pyrophosphate Metabolites by Vγ9Vδ2 T Cells
- PMID: 25657647
- PMCID: PMC4303140
- DOI: 10.3389/fimmu.2014.00688
Sensing of Pyrophosphate Metabolites by Vγ9Vδ2 T Cells
Abstract
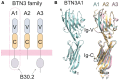
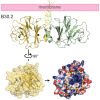
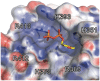
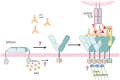
The predominant population of γδ T cells in human blood express a T cell receptor (TCR) composed of a Vγ9 (Vγ2 in an alternate nomenclature) and Vδ2 domains. These cells came into the limelight when it was discovered they can respond to certain microbial infections and tumorigenic cells through the detection of small, pyrophosphate containing organic molecules collectively called "phosphoantigens" or "pAgs." These molecules are intermediates in both eukaryotic and prokaryotic metabolic pathways. Chemical variants of these intermediates have been used in the clinic to treat a range of different cancers, however, directed optimization of these molecules requires a full understanding of their mechanism of action on target cells. We and others have identified a subclass of butyrophilin-related molecules (BTN3A1-3) that are directly involved in pAg sensing in the target cell, leading to engagement and activation of the T cell through the TCR. Our data and that of others support the pAg binding site to be the intracellular B30.2 domain of BTN3A1, which is the only isoform capable of mediating pAg-dependent stimulation of Vγ9Vδ2 T cells. Here, we review the data demonstrating pAg binding to the B30.2 domain and our studies of the structural conformations of the BTN3A extracellular domains. Finally, we synthesize a model linking binding of pAg to the intracellular domain with T cell detection via the extracellular domains in an "inside-out" signaling mechanism of the type characterized first for integrin molecule signaling. We also explore the role of Vγ9Vδ2 TCR variability in the CDR3 γ and δ loops and how this may modulate Vγ9Vδ2 cells as a population in surveillance of human health and disease.
Keywords: B30.2; T cell receptor; T cells; Vγ9Vδ2; butyrophilins; phosphoantigens.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

