Overexpression of copper/zinc superoxide dismutase from mangrove Kandelia candel in tobacco enhances salinity tolerance by the reduction of reactive oxygen species in chloroplast
- PMID: 25657655
- PMCID: PMC4302849
- DOI: 10.3389/fpls.2015.00023
Overexpression of copper/zinc superoxide dismutase from mangrove Kandelia candel in tobacco enhances salinity tolerance by the reduction of reactive oxygen species in chloroplast
Abstract
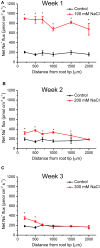
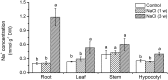
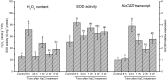
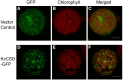
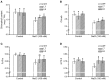
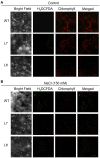
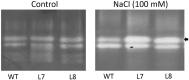
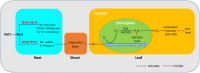
Na(+) uptake and transport in Kandelia candel and antioxidative defense were investigated under rising NaCl stress from 100 to 300 mM. Salinized K. candel roots had a net Na(+) efflux with a declined flux rate during an extended NaCl exposure. Na(+) buildup in leaves enhanced H2O2 levels, superoxide dismutase (SOD) activity, and increased transcription of CSD gene encoding a Cu/Zn SOD. Sequence and subcellular localization analyses have revealed that KcCSD is a typical Cu/Zn SOD in chloroplast. The transgenic tobacco experimental system was used as a functional genetics model to test the effect of KcCSD on salinity tolerance. KcCSD-transgenic lines were more Na(+) tolerant than wild-type (WT) tobacco in terms of lipid peroxidation, root growth, and survival rate. In the latter, 100 mM NaCl led to a remarkable reduction in chlorophyll content and a/b ratio, decreased maximal chlorophyll a fluorescence, and photochemical efficiency of photosystem II. NaCl stress in WT resulted from H2O2 burst in chloroplast. Na(+) injury to chloroplast was less pronounced in KcCSD-transgenic plants due to upregulated antioxidant defense. KcCSD-transgenic tobacco enhanced SOD activity by an increment in SOD isoenzymes under 100 mM NaCl stress from 24 h to 7 day. Catalase activity rose in KcCSD overexpressing tobacco plants. KcCSD-transgenic plants better scavenged NaCl-elicited reactive oxygen species (ROS) compared to WT ones. In conclusion, K. candel effectively excluded Na(+) in roots during a short exposure; and increased CSD expression to reduce ROS in chloroplast in a long-term and high saline environment.
Keywords: Kandelia candel; Na+ flux; catalase; hydrogen peroxide; salt; superoxide anion; superoxide dismutase.
Figures




References
-
- Aebi H. (1984). Catalase in vitro. Meth. Enzymol. 105, 121–126. - PubMed
-
- Bowler C., Montagu M. V., Inze D. (1992). Superoxide dismutase and stress tolerance. Ann. Rev. Plant Biol. 43, 83–116.
LinkOut - more resources
Full Text Sources
Other Literature Sources

