Interleukin-28B (rs12979860) gene variation and treatment outcome after peginterferon plus ribavirin therapy in patients with genotype 1 of hepatitis C virus
- PMID: 25657752
- PMCID: PMC4310080
Interleukin-28B (rs12979860) gene variation and treatment outcome after peginterferon plus ribavirin therapy in patients with genotype 1 of hepatitis C virus
Abstract
Background: The success of treatment of chronic hepatitis C (CHC) with pegylated interferon-α (PEG-IFN-α) and ribavirin (RBV) is affected by several host, viral, and treatment factors. This study was designed to describe the association of interleukin (IL) 28B genotypes for rs12979860 with sustained virologic response (SVR) in patients with genotype 1 CHC infection treated with PEG-IFN α-2 and RBV.
Materials and methods: Interleukin-28B genotype in 100 studied patients was detected by tagman real-time polymerase chain reaction. Before treatment blood samples were obtained, then patients were treated for 48-week with a combination therapy using of the PEG-IFN α-2 and RBV. SVR evaluated 6 months after stopping therapy, and was defined as undetectable plasma hepatitis C virus-RNA.
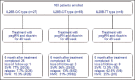
Results: Among studied patients, 65% were IL-28B CT, 27% CC, and 8% TT. In all studied patients, SVR was 58.3%, relapse 15.6%, and null virological response 26.1%. SVR rates were 76.9% in IL-28B-CC, 56.4% in IL-28B-CT, and 12.5% in IL-28B-TT patients. Relapse rates were 7.7% in IL-28B-CC, 12.9% in IL-28B-CT, and 62.5% in IL-28B-TT patients. There was a significant difference between response to treatment in patients IL-28B-CC, CT, and TT (P = 0.003). IL-28B genotype CC, (odds ratio = 0.053, 95% confidence interval; 0.005-0.54, P = 0.03), was the independent predicting factor.
Conclusion: Interleukin-28B was an important predictor of CHC treatment outcome with Peg-IFN-α and RBV. IL-28B-CC seems to be more important than IL-28B-CT/TT in predicting positive treatment response.
Keywords: Chronic hepatitis C; interleukin-28B; pegylated interferon-α; ribavirin.
Figures
References
-
- Ray Kim W. Global epidemiology and burden of hepatitis C. Microbes Infect. 2002;4:1219–25. - PubMed
-
- Alavian SM. We need a new national approach to control hepatitis C: It is becoming too late. Hepat Mon. 2008;8:165–9.
-
- Brown RS, Jr, Gaglio PJ. Scope of worldwide hepatitis C problem. Liver Transpl. 2003;9:S10–3. - PubMed
LinkOut - more resources
Full Text Sources